Medical Device-Associated Biofilm Infections and Multidrug-Resistant Pathogens
- PMID: 38787246
- PMCID: PMC11124157
- DOI: 10.3390/pathogens13050393
Medical Device-Associated Biofilm Infections and Multidrug-Resistant Pathogens
Abstract
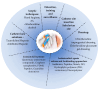
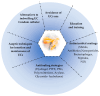
Medical devices such as venous catheters (VCs) and urinary catheters (UCs) are widely used in the hospital setting. However, the implantation of these devices is often accompanied by complications. About 60 to 70% of nosocomial infections (NIs) are linked to biofilms. The main complication is the ability of microorganisms to adhere to surfaces and form biofilms which protect them and help them to persist in the host. Indeed, by crossing the skin barrier, the insertion of VC inevitably allows skin flora or accidental environmental contaminants to access the underlying tissues and cause fatal complications like bloodstream infections (BSIs). In fact, 80,000 central venous catheters-BSIs (CVC-BSIs)-mainly occur in intensive care units (ICUs) with a death rate of 12 to 25%. Similarly, catheter-associated urinary tract infections (CA-UTIs) are the most commonlyhospital-acquired infections (HAIs) worldwide.These infections represent up to 40% of NIs.In this review, we present a summary of biofilm formation steps. We provide an overview of two main and important infections in clinical settings linked to medical devices, namely the catheter-asociated bloodstream infections (CA-BSIs) and catheter-associated urinary tract infections (CA-UTIs), and highlight also the most multidrug resistant bacteria implicated in these infections. Furthermore, we draw attention toseveral useful prevention strategies, and advanced antimicrobial and antifouling approaches developed to reduce bacterial colonization on catheter surfaces and the incidence of the catheter-related infections.
Keywords: biofilms; bloodstream infections; medical device; nosocomial infections; pathogens; urinary tract infections.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Santos F.K.Y., Flumignan R.L.G., Areias L.L., Sarpe A.K.P., Amaral F.C.F., Ávila R.B., Vasconcelos V.T., Guedes Neto H.J., Amorim J.E., Nakano L.C.U. Peripherally inserted central catheter versus central venous catheter for intravenous access: A protocol for systematic review and meta-analysis. Medicine. 2020;99:e20352. doi: 10.1097/MD.0000000000020352. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical