Improved Treatment Outcome Following the Use of a Wound Dressings in Cutaneous Leishmaniasis Lesions
- PMID: 38787268
- PMCID: PMC11124396
- DOI: 10.3390/pathogens13050416
Improved Treatment Outcome Following the Use of a Wound Dressings in Cutaneous Leishmaniasis Lesions
Abstract
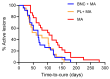
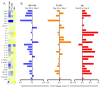
Leishmaniasis, caused by Leishmania parasites, is a neglected tropical disease and Cutaneous Leishmaniasis (CL) is the most common form. Despite the associated toxicity and adverse effects, Meglumine antimoniate (MA) remains the first-choice treatment for CL in Brazil, pressing the need for the development of better alternatives. Bacterial NanoCellulose (BNC), a biocompatible nanomaterial, has unique properties regarding wound healing. In a previous study, we showed that use of topical BNC + systemic MA significantly increased the cure rate of CL patients, compared to treatment with MA alone. Herein, we performed a study comparing the combination of a wound dressing (BNC or placebo) plus systemic MA versus systemic MA alone, in CL caused by Leishmania braziliensis. We show that patients treated with the combination treatment (BNC or placebo) + MA showed improved cure rates and decreased need for rescue treatment, although differences compared to controls (systemic MA alone) were not significant. However, the overall time-to-cure was significantly lower in groups treated with the combination treatment (BNC+ systemic MA or placebo + systemic MA) in comparison to controls (MA alone), indicating that the use of a wound dressing improves CL treatment outcome. Assessment of the immune response in peripheral blood showed an overall downmodulation in the inflammatory landscape and a significant decrease in the production of IL-1a (p < 0.05) in patients treated with topical BNC + systemic MA. Our results show that the application of wound dressings to CL lesions can improve chemotherapy outcome in CL caused by L. braziliensis.
Keywords: Leishmania braziliensis; meglumine antimoniate; skin lesion; topical therapy; wound dressing.
Conflict of interest statement
The authors declare no conflicts of interest. Seven Biotecnologia-Nexfill had no role in study design, analysis, decision to publish, or preparation of the manuscript.
Figures

Similar articles
-
Use of topical rSm29 in combination with intravenous meglumine antimoniate in the treatment of cutaneous leishmaniasis: A randomized controlled trial.Int J Infect Dis. 2024 Oct;147:107206. doi: 10.1016/j.ijid.2024.107206. Epub 2024 Aug 13. Int J Infect Dis. 2024. PMID: 39147194 Clinical Trial.
-
Dressings combined with injection of meglumine antimoniate in the treatment of cutaneous leishmaniasis: a randomized controlled clinical trial.PLoS One. 2013 Jun 24;8(6):e66123. doi: 10.1371/journal.pone.0066123. Print 2013. PLoS One. 2013. PMID: 23826087 Free PMC article. Clinical Trial.
-
Pentavalent Antimony Associated with G-CSF in the Treatment of Cutaneous Leishmaniasis Caused by Leishmania (Viannia) braziliensis.Pathogens. 2024 Apr 4;13(4):301. doi: 10.3390/pathogens13040301. Pathogens. 2024. PMID: 38668256 Free PMC article.
-
American tegumentary leishmaniasis in Brazil: a critical review of the current therapeutic approach with systemic meglumine antimoniate and short-term possibilities for an alternative treatment.Trop Med Int Health. 2019 Apr;24(4):380-391. doi: 10.1111/tmi.13210. Epub 2019 Feb 19. Trop Med Int Health. 2019. PMID: 30681239 Review.
-
Disseminated Leishmaniasis, a Severe Form of Leishmania braziliensis Infection.Emerg Infect Dis. 2024 Mar;30(3):510-518. doi: 10.3201/eid3003.230786. Emerg Infect Dis. 2024. PMID: 38407142 Free PMC article.
References
-
- Leishmaniasis—PAHO/WHO|Pan American Health Organization. [(accessed on 18 December 2023)]. Available online: https://www.paho.org/en/topics/leishmaniasis.
-
- Belo V.S., Bruhn F.R.P., Barbosa D.S., Câmara D.C.P., Simões T.C., Buzanovsky L.P., Duarte A.G.S., De Melo S.N., Cardoso D.T., Donato L.E., et al. Temporal Patterns, Spatial Risks, and Characteristics of Tegumentary Leishmaniasis in Brazil in the First Twenty Years of the 21st Century. PLoS Negl. Trop. Dis. 2023;17:e0011405. doi: 10.1371/journal.pntd.0011405. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

