Innervation of adipocytes is limited in mouse perivascular adipose tissue
- PMID: 38787382
- PMCID: PMC11380956
- DOI: 10.1152/ajpheart.00041.2024
Innervation of adipocytes is limited in mouse perivascular adipose tissue
Abstract
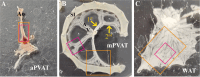
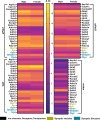
Perivascular adipose tissue (PVAT) regulates vascular tone by releasing anticontractile factors. These anticontractile factors are driven by processes downstream of adipocyte stimulation by norepinephrine; however, whether norepinephrine originates from neural innervation or other sources is unknown. The goal of this study was to test the hypothesis that neurons innervating PVAT provide the adrenergic drive to stimulate adipocytes in aortic and mesenteric perivascular adipose tissue (aPVAT and mPVAT), and white adipose tissue (WAT). Healthy male and female mice (8-13 wk) were used in all experiments. Expression of genes associated with synaptic transmission were quantified by qPCR and adipocyte activity in response to neurotransmitters and neuron depolarization was assessed in AdipoqCre+;GCaMP5g-tdTf/WT mice. Immunostaining, tissue clearing, and transgenic reporter lines were used to assess anatomical relationships between nerves and adipocytes. Although synaptic transmission component genes are expressed in adipose tissues (aPVAT, mPVAT, and WAT), strong nerve stimulation with electrical field stimulation does not significantly trigger calcium responses in adipocytes. However, norepinephrine consistently elicits strong calcium responses in adipocytes from all adipose tissues studied. Bethanechol induces minimal adipocyte responses. Imaging neural innervation using various techniques reveals that nerve fibers primarily run alongside blood vessels and rarely branch into the adipose tissue. Although nerve fibers are associated with blood vessels in adipose tissue, they demonstrate limited anatomical and functional interactions with adjacent adipocytes, challenging the concept of classical innervation. These findings dispute the significant involvement of neural input in regulating PVAT adipocyte function and emphasize alternative mechanisms governing adrenergic-driven anticontractile functions of PVAT.NEW & NOTEWORTHY This study challenges prevailing views on neural innervation in perivascular adipose tissue (PVAT) and its role in adrenergic-driven anticontractile effects on vasculature. Contrary to existing paradigms, limited anatomical and functional connections were found between PVAT nerve fibers and adipocytes, underscoring the importance of exploring alternative mechanistic pathways. Understanding the mechanisms involved in PVAT's anticontractile effects is critical for developing potential therapeutic interventions against dysregulated vascular tone, hypertension, and cardiovascular disease.
Keywords: adipocytes; innervation; perivascular adipose tissue.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures












Comment in
-
Nervy issues of fatty tissues: a deeper dig into the innervation of fat.Am J Physiol Heart Circ Physiol. 2024 Oct 1;327(4):H826-H827. doi: 10.1152/ajpheart.00355.2024. Epub 2024 Aug 23. Am J Physiol Heart Circ Physiol. 2024. PMID: 39178025 No abstract available.
Similar articles
-
Role of Sympathetic Nerves and Adipocyte Catecholamine Uptake in the Vasorelaxant Function of Perivascular Adipose Tissue.Arterioscler Thromb Vasc Biol. 2018 Apr;38(4):880-891. doi: 10.1161/ATVBAHA.118.310777. Epub 2018 Mar 1. Arterioscler Thromb Vasc Biol. 2018. PMID: 29496660
-
Endothelin-1- and acetylcholine-mediated effects in human and rat vessels: impact of perivascular adipose tissue, diabetes, angiotensin II, and chemerin.Blood Press. 2024 Dec;33(1):2414072. doi: 10.1080/08037051.2024.2414072. Epub 2024 Oct 10. Blood Press. 2024. PMID: 39387176
-
β3 -Adrenoceptor stimulation of perivascular adipocytes leads to increased fat cell-derived NO and vascular relaxation in small arteries.Br J Pharmacol. 2018 Sep;175(18):3685-3698. doi: 10.1111/bph.14433. Epub 2018 Aug 10. Br J Pharmacol. 2018. PMID: 29980164 Free PMC article.
-
New actions of an old friend: perivascular adipose tissue's adrenergic mechanisms.Br J Pharmacol. 2017 Oct;174(20):3454-3465. doi: 10.1111/bph.13663. Epub 2016 Dec 4. Br J Pharmacol. 2017. PMID: 27813085 Free PMC article. Review.
-
Perivascular Adipose Tissue: the Sixth Man of the Cardiovascular System.Cardiovasc Drugs Ther. 2018 Oct;32(5):481-502. doi: 10.1007/s10557-018-6820-z. Cardiovasc Drugs Ther. 2018. PMID: 30171461 Free PMC article. Review.
Cited by
-
Perivascular adipose tissue: a central player in the triad of diabetes, obesity, and cardiovascular health.Cardiovasc Diabetol. 2024 Dec 28;23(1):455. doi: 10.1186/s12933-024-02549-9. Cardiovasc Diabetol. 2024. PMID: 39732729 Free PMC article. Review.
-
Sympathetic nerves are sparsely distributed in rat mesenteric perivascular adipose tissue.Front Physiol. 2025 May 23;16:1547785. doi: 10.3389/fphys.2025.1547785. eCollection 2025. Front Physiol. 2025. PMID: 40488143 Free PMC article.
-
Sex-Specific Characteristics of Perivascular Fat in Aortic Aneurysms.J Clin Med. 2025 Apr 29;14(9):3071. doi: 10.3390/jcm14093071. J Clin Med. 2025. PMID: 40364103 Free PMC article. Review.
-
Rat perivascular adipose tissue microvasculature revealed by tissue clearing.Front Physiol. 2025 Jan 31;15:1535711. doi: 10.3389/fphys.2024.1535711. eCollection 2024. Front Physiol. 2025. PMID: 39959814 Free PMC article.
References
-
- Eldholm RS, Persson K, Barca ML, Knapskog A-B, Cavallin L, Engedal K, Selbaek G, Skovlund E, Saltvedt I. Association between vascular comorbidity and progression of Alzheimer’s disease: a two-year observational study in Norwegian memory clinics. BMC Geriatr 18: 120, 2018. doi: 10.1186/s12877-018-0813-4. - DOI - PMC - PubMed
-
- Lorius N, Locascio JJ, Rentz DM, Johnson KA, Sperling RA, Viswanathan A, Marshall GA; Alzheimer’s Disease Neuroimaging Initiative. Vascular disease and risk factors are associated with cognitive decline in the Alzheimer’s disease spectrum. Alzheimer Dis Assoc Disord 29: 18–25, 2015. doi: 10.1097/WAD.0000000000000043. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

