PI-QUAL version 2: an update of a standardised scoring system for the assessment of image quality of prostate MRI
- PMID: 38787428
- PMCID: PMC11519155
- DOI: 10.1007/s00330-024-10795-4
PI-QUAL version 2: an update of a standardised scoring system for the assessment of image quality of prostate MRI
Abstract
Multiparametric MRI is the optimal primary investigation when prostate cancer is suspected, and its ability to rule in and rule out clinically significant disease relies on high-quality anatomical and functional images. Avenues for achieving consistent high-quality acquisitions include meticulous patient preparation, scanner setup, optimised pulse sequences, personnel training, and artificial intelligence systems. The impact of these interventions on the final images needs to be quantified. The prostate imaging quality (PI-QUAL) scoring system was the first standardised quantification method that demonstrated the potential for clinical benefit by relating image quality to cancer detection ability by MRI. We present the updated version of PI-QUAL (PI-QUAL v2) which applies to prostate MRI performed with or without intravenous contrast medium using a simplified 3-point scale focused on critical technical and qualitative image parameters. CLINICAL RELEVANCE STATEMENT: High image quality is crucial for prostate MRI, and the updated version of the PI-QUAL score (PI-QUAL v2) aims to address the limitations of version 1. It is now applicable to both multiparametric MRI and MRI without intravenous contrast medium. KEY POINTS: High-quality images are essential for prostate cancer diagnosis and management using MRI. PI-QUAL v2 simplifies image assessment and expands its applicability to prostate MRI without contrast medium. PI-QUAL v2 focuses on critical technical and qualitative image parameters and emphasises T2-WI and DWI.
Keywords: Magnetic resonance imaging; Prostatic neoplasms; Quality control.
© 2024. The Author(s).
Conflict of interest statement
Mark Emberton receives research support from the United Kingdom’s National Institute of Health Research (NIHR) UCLH/UCL Biomedical Research Centre. He was awarded NIHR Senior Investigator status in 2015. He acts as consultant/adviser to Sonacare Inc., Angiodynamics Inc., Profound Medical Inc., Exact Imaging Inc. and Early Health Ltd. Francesco Giganti receives consulting fees from Lucida Medical Ltd outside of the submitted work. Valeria Panebianco is a member of the Scientific Editorial Board for European Radiology (Urogenital Section). As such, she had no role in handling the manuscript or finalising decisions. The remaining authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Figures

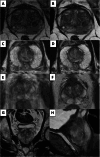
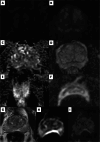

Comment in
-
PI-QUAL version 2: the urologist's perspective.Eur Radiol. 2024 Nov;34(11):7063-7064. doi: 10.1007/s00330-024-10844-y. Epub 2024 Jun 21. Eur Radiol. 2024. PMID: 38904759 No abstract available.
-
PI-QUAL version 2: the radiologist's perspective.Eur Radiol. 2024 Nov;34(11):7065-7067. doi: 10.1007/s00330-024-10845-x. Epub 2024 Jun 24. Eur Radiol. 2024. PMID: 38913247 No abstract available.
References
-
- van der Leest M, Cornel E, Israel B et al (2019) Head-to-head comparison of transrectal ultrasound-guided prostate biopsy versus multiparametric prostate resonance imaging with subsequent magnetic resonance-guided biopsy in biopsy-naive men with elevated prostate-specific antigen: a large prospective multicenter clinical study. Eur Urol 75:570–578 - PubMed
-
- Rouvière O, Puech P, Renard-Penna R et al (2019) Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy-naive patients (MRI-FIRST): a prospective, multicentre, paired diagnostic study. Lancet Oncol 20:100–109 - PubMed
-
- Kasivisvanathan V, Stabile A, Neves JB et al (2019) Magnetic resonance imaging-targeted biopsy versus systematic biopsy in the detection of prostate cancer: a systematic review and meta-analysis. Eur Urol 76:284–303 - PubMed
-
- Ahmed HU, El-Shater Bosaily A, Brown LC et al (2017) Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 389:815–822 - PubMed
-
- Mottet N, van den Bergh RCN, Briers E et al (2021) EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer-2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol 79:243–262 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

