Low frequency of Vγ9Vδ2 T cells predicts poor survival in newly diagnosed acute myeloid leukemia
- PMID: 38788176
- PMCID: PMC11372596
- DOI: 10.1182/bloodadvances.2023011594
Low frequency of Vγ9Vδ2 T cells predicts poor survival in newly diagnosed acute myeloid leukemia
Abstract
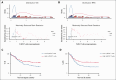
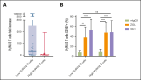
In several tumor subtypes, an increased infiltration of Vγ9Vδ2 T cells has been shown to have the highest prognostic value compared with other immune subsets. In acute myeloid leukemia (AML), similar findings have been based solely on the inference of transcriptomic data and have not been assessed with respect to confounding factors. This study aimed at determining, by immunophenotypic analysis (flow or mass cytometry) of peripheral blood from patients with AML at diagnosis, the prognostic impact of Vγ9Vδ2 T-cell frequency. This was adjusted for potential confounders (age at diagnosis, disease status, European LeukemiaNet classification, leukocytosis, and allogeneic hematopoietic stem cell transplantation as a time-dependent covariate). The cohort was composed of 198 patients with newly diagnosed (ND) AML. By univariate analysis, patients with lower Vγ9Vδ2 T cells at diagnosis had significantly lower 5-year overall and relapse-free survivals. These results were confirmed in multivariate analysis (hazard ratio [HR], 1.55 [95% confidence interval (CI), 1.04-2.30]; P = .030 and HR, 1.64 [95% CI, 1.06-2.53]; P = .025). Immunophenotypic alterations observed in patients with lower Vγ9Vδ2 T cells included a loss of some cytotoxic Vγ9Vδ2 T-cell subsets and a decreased expression of butyrophilin 3A on the surface of blasts. Samples expanded regardless of their Vγ9Vδ2 T-cell levels and displayed similar effector functions in vitro. This study confirms the prognostic value of elevated Vγ9Vδ2 T cells among lymphocytes in patients with ND AML. These results provide a strong rationale to consider consolidation protocols aiming at enhancing Vγ9Vδ2 T-cell responses.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: D.O. is a cofounder and shareholder of ImCheck Therapeutics. The remaining authors declare no competing financial interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

