Synchrotron Near-Field Infrared Nanospectroscopy and Nanoimaging of Lithium Fluoride in Solid Electrolyte Interphases in Li-Ion Battery Anodes
- PMID: 38788214
- PMCID: PMC11171761
- DOI: 10.1021/acsnano.4c04333
Synchrotron Near-Field Infrared Nanospectroscopy and Nanoimaging of Lithium Fluoride in Solid Electrolyte Interphases in Li-Ion Battery Anodes
Abstract
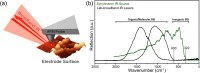
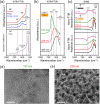
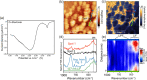
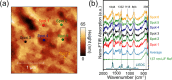
Lithium fluoride (LiF) is a ubiquitous component in the solid electrolyte interphase (SEI) layer in Li-ion batteries. However, its nanoscale structure, morphology, and topology, important factors for understanding LiF and SEI film functionality, including electrode passivity, are often unknown due to limitations in spatial resolution of common characterization techniques. Ultrabroadband near-field synchrotron infrared nanospectroscopy (SINS) enables such detection and mapping of LiF in SEI layers in the far-infrared region down to ca. 322 cm-1 with a nanoscale spatial resolution of ca. 20 nm. The surface sensitivity of SINS and the large infrared absorption cross section of LiF, which can support local surface phonons under certain circumstances, enabled characterization of model LiF samples of varying structure, thickness, surface roughness, and degree of crystallinity, as confirmed by atomic force microscopy, attenuated total reflectance FTIR, SINS, X-ray photoelectron spectroscopy, high-angle annular dark-field, and scanning transmission electron microscopy. Enabled by this approach, LiF within SEI films formed on Cu, Si, and metallic glass Si40Al50Fe10 electrodes was detected and characterized. The nanoscale morphologies and topologies of LiF in these SEI layers were evaluated to gain insights into LiF nucleation, growth, and the resulting nuances in the electrode surface passivity.
Keywords: LiF; SEI; SINS; anode; interface; interphase; nano-FTIR.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- McBrayer J. D.; Rodrigues M. T. F.; Schulze M. C.; Abraham D. P.; Apblett C. A.; Bloom I.; Carroll G. M.; Colclasure A. M.; Fang C.; Harrison K. L.; Liu G.; Minteer S. D.; Neale N. R.; Veith G. M.; Johnson C. S.; Vaughey J. T.; Burrell A. K.; Cunningham B. Calendar Aging of Silicon-Containing Batteries. Nat. Energy 2021, 6 (9), 866–872. 10.1038/s41560-021-00883-w. - DOI
-
- Schulze M. C.; Rodrigues M.-T. F.; McBrayer J. D.; Abraham D. P.; Apblett C. A.; Bloom I.; Chen Z.; Colclasure A. M.; Dunlop A. R.; Fang C.; Harrison K. L.; Liu G.; Minteer S. D.; Neale N. R.; Robertson D.; Tornheim A. P.; Trask S. E.; Veith G. M.; Verma A.; Yang Z.; Johnson C. Critical Evaluation of Potentiostatic Holds as Accelerated Predictors of Capacity Fade during Calendar Aging. J. Electrochem. Soc. 2022, 169 (5), 05053110.1149/1945-7111/ac6f88. - DOI
-
- Peled E.; Menkin S. Review—SEI: Past, Present and Future. J. Electrochem. Soc. 2017, 164 (7), A1703–A1719. 10.1149/2.1441707jes. - DOI

