Targeted rescue of synaptic plasticity improves cognitive decline in sepsis-associated encephalopathy
- PMID: 38788710
- PMCID: PMC11286813
- DOI: 10.1016/j.ymthe.2024.05.001
Targeted rescue of synaptic plasticity improves cognitive decline in sepsis-associated encephalopathy
Abstract
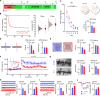
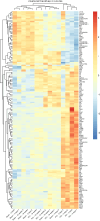
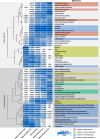
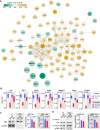
Sepsis-associated encephalopathy (SAE) is a frequent complication of severe systemic infection resulting in delirium, premature death, and long-term cognitive impairment. We closely mimicked SAE in a murine peritoneal contamination and infection (PCI) model. We found long-lasting synaptic pathology in the hippocampus including defective long-term synaptic plasticity, reduction of mature neuronal dendritic spines, and severely affected excitatory neurotransmission. Genes related to synaptic signaling, including the gene for activity-regulated cytoskeleton-associated protein (Arc/Arg3.1) and members of the transcription-regulatory EGR gene family, were downregulated. At the protein level, ARC expression and mitogen-activated protein kinase signaling in the brain were affected. For targeted rescue we used adeno-associated virus-mediated overexpression of ARC in the hippocampus in vivo. This recovered defective synaptic plasticity and improved memory dysfunction. Using the enriched environment paradigm as a non-invasive rescue intervention, we found improvement of defective long-term potentiation, memory, and anxiety. The beneficial effects of an enriched environment were accompanied by an increase in brain-derived neurotrophic factor (BDNF) and ARC expression in the hippocampus, suggesting that activation of the BDNF-TrkB pathway leads to restoration of the PCI-induced reduction of ARC. Collectively, our findings identify synaptic pathomechanisms underlying SAE and provide a conceptual approach to target SAE-induced synaptic dysfunction with potential therapeutic applications to patients with SAE.
Keywords: AAV-mediated overexpression; ARC; BDNF; enriched environment; hippocampus; memory dysfunction; sepsis-associated encephalopathy; synaptic plasticity.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
-
- Sipila P.N., Heikkila N., Lindbohm J.V., Hakulinen C., Vahtera J., Elovainio M., Suominen S., Vaananen A., Koskinen A., Nyberg S.T., et al. Hospital-treated infectious diseases and the risk of dementia: a large, multicohort, observational study with a replication cohort. Lancet Infect. Dis. 2021;21:1557–1567. - PMC - PubMed
-
- Fleischmann C., Scherag A., Adhikari N.K.J., Hartog C.S., Tsaganos T., Schlattmann P., Angus D.C., Reinhart K., International Forum of Acute Care Trialists Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am. J. Respir. Crit. Care Med. 2016;193:259–272. - PubMed
-
- Ely E.W., Shintani A., Truman B., Speroff T., Gordon S.M., Harrell F.E., Jr., Inouye S.K., Bernard G.R., Dittus R.S. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–1762. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

