Breadth of Fc-mediated effector function correlates with clinical immunity following human malaria challenge
- PMID: 38788711
- PMCID: PMC7616646
- DOI: 10.1016/j.immuni.2024.05.001
Breadth of Fc-mediated effector function correlates with clinical immunity following human malaria challenge
Abstract
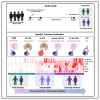
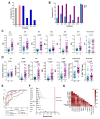
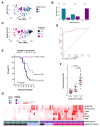
Malaria is a life-threatening disease of global health importance, particularly in sub-Saharan Africa. The growth inhibition assay (GIA) is routinely used to evaluate, prioritize, and quantify the efficacy of malaria blood-stage vaccine candidates but does not reliably predict either naturally acquired or vaccine-induced protection. Controlled human malaria challenge studies in semi-immune volunteers provide an unparalleled opportunity to robustly identify mechanistic correlates of protection. We leveraged this platform to undertake a head-to-head comparison of seven functional antibody assays that are relevant to immunity against the erythrocytic merozoite stage of Plasmodium falciparum. Fc-mediated effector functions were strongly associated with protection from clinical symptoms of malaria and exponential parasite multiplication, while the gold standard GIA was not. The breadth of Fc-mediated effector function discriminated clinical immunity following the challenge. These findings present a shift in the understanding of the mechanisms that underpin immunity to malaria and have important implications for vaccine development.
Keywords: CHMI; Fc-mediated effector function; antibodies; growth inhibition activity; malaria; merozoite; naturally acquired immunity; plasmodium falciparum.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests In the CHMI-SIKA team, Y.A., P.F.B., S.L.H., E.R.J., B.K.L.S., and T.L.R. are salaried, full-time employees of Sanaria Inc., the manufacturer of Sanaria PfSPZ Challenge. Thus, all authors associated with Sanaria Inc. have potential conflicts of interest.
Figures

References
-
- Phillips MA, Burrows JN, Manyando C, van Huijsduijnen RH, Van Voorhis WC, Wells TNC. Malaria. Nat Rev Dis Primers. 2017;3:17050. - PubMed
-
- Cowman AF, Healer J, Marapana D, Marsh K. Malaria: Biology and Disease. Cell. 2016;167:610–624. - PubMed
-
- WHO. World Malaria Report. World Health Organization; 2023.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

