IL-27 promotes pathogenic T cells in a mouse model of Sjögren's disease
- PMID: 38788885
- PMCID: PMC11203157
- DOI: 10.1016/j.clim.2024.110260
IL-27 promotes pathogenic T cells in a mouse model of Sjögren's disease
Abstract
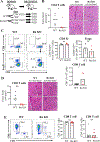
Sjögren's disease (SjD) is a chronic autoimmune disease characterized by focal lymphocytic inflammation in lacrimal and salivary glands. We recently identified IL-27 as a requisite signal for the spontaneous SjD-like manifestations in nonobese diabetic (NOD) mice. Here, we define T cell-intrinsic effects of IL-27 in lacrimal gland disease in NOD mice. IL-27 receptor was required by both CD4 T effector (Te) cells and CD8 T cells to mediate focal inflammation. Intrinsic IL-27 signaling was associated with PD-1 and ICOS expressing T follicular helper (Tfh)-like CD4 Te cells within lacrimal glands, including subsets defined by CD73 or CD39 expression. CD8 T cells capable of IL-27 signaling also expressed PD-1 with subsets expressing ICOS and CD73 demonstrating a T follicular cytotoxic (Tfc)-like cell phenotype and others expressing a CD39hi exhausted-like phenotype. These findings suggest IL-27 is a key early signal driving a follicular-type response in lacrimal gland inflammation in NOD mice.
Keywords: Exhausted CD8 T cells; IL-27; Sjögren's disease; T follicular cytotoxic cells; T follicular helper cells; T peripheral helper cells.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest All authors declare that no conflicts of interest exist.
Figures




References
-
- Vivino FB, Bunya VY, Massaro-Giordano G, Johr CR, Giattino SL, Schorpion A et al. Sjogren’s syndrome: An update on disease pathogenesis, clinical manifestations and treatment. Clinical immunology (Orlando, Fla) 2019;203:81–121. - PubMed
-
- Yao Y, Ma J-F, Chang C, Xu T, Gao C-Y, Gershwin ME et al. Immunobiology of T cells in Sjögren’s syndrome. Clinical Reviews in Allergy & Immunology 2021;60:111–31. - PubMed
-
- Adamson T 3rd, Fox R, Frisman D, Howell F. Immunohistologic analysis of lymphoid infiltrates in primary Sjogren’s syndrome using monoclonal antibodies. Journal of immunology (Baltimore, Md: 1950) 1983;130:203–8. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

