Deleting the mitochondrial respiration negative regulator MCJ enhances the efficacy of CD8+ T cell adoptive therapies in pre-clinical studies
- PMID: 38789421
- PMCID: PMC11126743
- DOI: 10.1038/s41467-024-48653-y
Deleting the mitochondrial respiration negative regulator MCJ enhances the efficacy of CD8+ T cell adoptive therapies in pre-clinical studies
Abstract
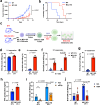
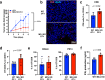
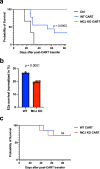
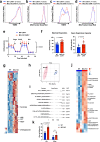
Mitochondrial respiration is essential for the survival and function of T cells used in adoptive cellular therapies. However, strategies that specifically enhance mitochondrial respiration to promote T cell function remain limited. Here, we investigate methylation-controlled J protein (MCJ), an endogenous negative regulator of mitochondrial complex I expressed in CD8 cells, as a target for improving the efficacy of adoptive T cell therapies. We demonstrate that MCJ inhibits mitochondrial respiration in murine CD8+ CAR-T cells and that deletion of MCJ increases their in vitro and in vivo efficacy against murine B cell leukaemia. Similarly, MCJ deletion in ovalbumin (OVA)-specific CD8+ T cells also increases their efficacy against established OVA-expressing melanoma tumors in vivo. Furthermore, we show for the first time that MCJ is expressed in human CD8 cells and that the level of MCJ expression correlates with the functional activity of CD8+ CAR-T cells. Silencing MCJ expression in human CD8 CAR-T cells increases their mitochondrial metabolism and enhances their anti-tumor activity. Thus, targeting MCJ may represent a potential therapeutic strategy to increase mitochondrial metabolism and improve the efficacy of adoptive T cell therapies.
© 2024. The Author(s).
Conflict of interest statement
M.R. declares to be a member of the scientific advisory board and co-founder of Mitotherapeutix, and is co-inventor in patents held by the University of Vermont. M.R., M.E.K., M.W. and M.C.Y. declare the filing of a patent that discloses findings described in this manuscript. R.A.F. is an advisor to Glaxo Smith Kline, Celsius, EvolveImmune, Ventus Therapeutics, and is the recipient of a grant from Genetech/Roche. The remaining authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
- R01 CA260909/CA/NCI NIH HHS/United States
- AI149187/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- 634956/Hyundai Motor Group | Hyundai Motor America | Hyundai Hope On Wheels (Hope On Wheels)
- P30CA046934/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- P30 DK045735/DK/NIDDK NIH HHS/United States
- CA223389/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- CA260909/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- AI148434/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- R21 CA223389/CA/NCI NIH HHS/United States
- CA086913/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- R21 AI149187/AI/NIAID NIH HHS/United States
- R21 CA127099/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

