A new mechanism of thyroid hormone receptor β agonists ameliorating nonalcoholic steatohepatitis by inhibiting intestinal lipid absorption via remodeling bile acid profiles
- PMID: 38789494
- PMCID: PMC11420233
- DOI: 10.1038/s41401-024-01303-x
A new mechanism of thyroid hormone receptor β agonists ameliorating nonalcoholic steatohepatitis by inhibiting intestinal lipid absorption via remodeling bile acid profiles
Abstract
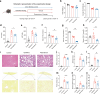
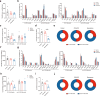
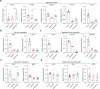
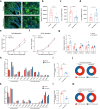
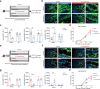
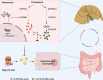
Excessive dietary calories lead to systemic metabolic disorders, disturb hepatic lipid metabolism, and aggravate nonalcoholic steatohepatitis (NASH). Bile acids (BAs) play key roles in regulating nutrition absorption and systemic energy homeostasis. Resmetirom is a selective thyroid hormone receptor β (THRβ) agonist and the first approved drug for NASH treatment. It is well known that the THRβ activation could promote intrahepatic lipid catabolism and improve mitochondrial function, however, its effects on intestinal lipid absorption and BA compositions remain unknown. In the present study, the choline-deficient, L-amino acid defined, high-fat diet (CDAHFD) and high-fat diet plus CCl4 (HFD+CCl4)-induced NASH mice were used to evaluate the effects of resmetirom on lipid and BA composition. We showed that resmetirom administration (10 mg·kg-1·d-1, i.g.) significantly altered hepatic lipid composition, especially reduced the C18:2 fatty acyl chain-containing triglyceride (TG) and phosphatidylcholine (PC) in the two NASH mouse models, suggesting that THRβ activation inhibited intestinal lipid absorption since C18:2 fatty acid could be obtained only from diet. Targeted analysis of BAs showed that resmetirom treatment markedly reduced the hepatic and intestinal 12-OH to non-12-OH BAs ratio by suppressing cytochrome P450 8B1 (CYP8B1) expression in both NASH mouse models. The direct inhibition by resmetirom on intestinal lipid absorption was further verified by the BODIPY gavage and the oral fat tolerance test. In addition, disturbance of the altered BA profiles by exogenous cholic acid (CA) supplementation abolished the inhibitory effects of resmetirom on intestinal lipid absorption in both normal and CDAHFD-fed mice, suggesting that resmetirom inhibited intestinal lipid absorption by reducing 12-OH BAs content. In conclusion, we discovered a novel mechanism of THRβ agonists on NASH treatment by inhibiting intestinal lipid absorption through remodeling BAs composition, which highlights the multiple regulation of THRβ activation on lipid metabolism and extends the current knowledge on the action mechanisms of THRβ agonists in NASH treatment.
Keywords: CYP8B1; THRβ agonists; bile acids composition; intestinal lipid absorption; nonalcoholic steatohepatitis; resmetirom.
© 2024. The Author(s), under exclusive licence to Shanghai Institute of Materia Medica, Chinese Academy of Sciences and Chinese Pharmacological Society.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Hodson L, Gunn PJ. The regulation of hepatic fatty acid synthesis and partitioning: the effect of nutritional state. Nat Rev Endocrinol. 2019;15:689–700. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

