Choosing a camera and optimizing system parameters for speckle contrast optical spectroscopy
- PMID: 38789499
- PMCID: PMC11126420
- DOI: 10.1038/s41598-024-62106-y
Choosing a camera and optimizing system parameters for speckle contrast optical spectroscopy
Abstract
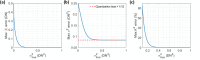
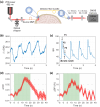
Speckle contrast optical spectroscopy (SCOS) is an emerging camera-based technique that can measure human cerebral blood flow (CBF) with high signal-to-noise ratio (SNR). At low photon flux levels typically encountered in human CBF measurements, camera noise and nonidealities could significantly impact SCOS measurement SNR and accuracy. Thus, a guide for characterizing, selecting, and optimizing a camera for SCOS measurements is crucial for the development of next-generation optical devices for monitoring human CBF and brain function. Here, we provide such a guide and illustrate it by evaluating three commercially available complementary metal-oxide-semiconductor cameras, considering a variety of factors including linearity, read noise, and quantization distortion. We show that some cameras that are well-suited for general intensity imaging could be challenged in accurately quantifying spatial contrast for SCOS. We then determine the optimal operating parameters for the preferred camera among the three and demonstrate measurement of human CBF with this selected low-cost camera. This work establishes a guideline for characterizing and selecting cameras as well as for determining optimal parameters for SCOS systems.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Bouma GJ, Muizelaar JP. Cerebral blood flow, cerebral blood volume, and cerebrovascular reactivity after severe head injury. J. Neurotrauma. 1992;9(Suppl 1):S333–348. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

