Phosphorus deficiency alleviates iron limitation in Synechocystis cyanobacteria through direct PhoB-mediated gene regulation
- PMID: 38789507
- PMCID: PMC11126600
- DOI: 10.1038/s41467-024-48847-4
Phosphorus deficiency alleviates iron limitation in Synechocystis cyanobacteria through direct PhoB-mediated gene regulation
Abstract
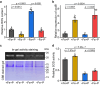
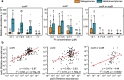
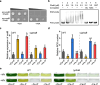
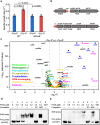
Iron and phosphorus are essential nutrients that exist at low concentrations in surface waters and may be co-limiting resources for phytoplankton growth. Here, we show that phosphorus deficiency increases the growth of iron-limited cyanobacteria (Synechocystis sp. PCC 6803) through a PhoB-mediated regulatory network. We find that PhoB, in addition to its well-recognized role in controlling phosphate homeostasis, also regulates key metabolic processes crucial for iron-limited cyanobacteria, including ROS detoxification and iron uptake. Transcript abundances of PhoB-targeted genes are enriched in samples from phosphorus-depleted seawater, and a conserved PhoB-binding site is widely present in the promoters of the target genes, suggesting that the PhoB-mediated regulation may be highly conserved. Our findings provide molecular insights into the responses of cyanobacteria to simultaneous iron/phosphorus nutrient limitation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Moore CM, et al. Processes and patterns of oceanic nutrient limitation. Nat. Geosci. 2013;6:701–710. doi: 10.1038/ngeo1765. - DOI
-
- Duhamel S, et al. Phosphorus as an integral component of global marine biogeochemistry. Nat. Geosci. 2021;14:359–368. doi: 10.1038/s41561-021-00755-8. - DOI
-
- Shaked Y, et al. Co-acquisition of mineral-bound iron and phosphorus by natural Trichodesmium colonies. Limnol. Oceanogr. 2023;5:1064–1077. doi: 10.1002/lno.12329. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

