Traumatic brain injury heterogeneity affects cell death and autophagy
- PMID: 38789796
- PMCID: PMC12414495
- DOI: 10.1007/s00221-024-06856-1
Traumatic brain injury heterogeneity affects cell death and autophagy
Abstract
Traumatic brain injury (TBI) mechanism and severity are heterogenous clinically, resulting in a multitude of physical, cognitive, and behavioral deficits. Impact variability influences the origin, spread, and classification of molecular dysfunction which limits strategies for comprehensive clinical intervention. Indeed, there are currently no clinically approved therapeutics for treating the secondary consequences associated with TBI. Thus, examining pathophysiological changes from heterogeneous impacts is imperative for improving clinical translation and evaluating the efficacy of potential therapeutic strategies. Here we utilized TBI models that varied in both injury mechanism and severity including severe traditional controlled cortical impact (CCI), modified mild CCI (MTBI), and multiple severities of closed-head diffuse TBI (DTBI), and assessed pathophysiological changes. Severe CCI induced cortical lesions and necrosis, while both MTBI and DTBI lacked lesions or significant necrotic damage. Autophagy was activated in the ipsilateral cortex following CCI, but acutely impaired in the ipsilateral hippocampus. Additionally, autophagy was activated in the cortex following DTBI, and autophagic impairment was observed in either the cortex or hippocampus following impact from each DTBI severity. Thus, we provide evidence that autophagy is a therapeutic target for both mild and severe TBI. However, dramatic increases in necrosis following CCI may negatively impact the clinical translatability of therapeutics designed to treat acute dysfunction in TBI. Overall, these results provide evidence that injury sequalae affiliated with TBI heterogeneity is linked through autophagy activation and/or impaired autophagic flux. Thus, therapeutic strategies designed to intervene in autophagy may alleviate pathophysiological consequences, in addition to the cognitive and behavioral deficits observed in TBI.
Keywords: Autophagy; Cell death; Concussion/mild TBI; Secondary injury.
© 2024. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
Statements and Declarations
The authors declare no conflict of interest. All animal work reported here was approved by the Institutional Animal Care and Use Committee at the University of Nebraska – Lincoln under protocol number 2300.
Figures
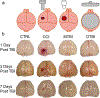


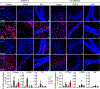
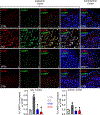
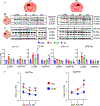
References
-
- Bolte S, & Cordelières FP (2006). A guided tour into subcellular colocalization analysis in light microscopy. Journal of microscopy, 224(3), 213–232. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

