heredERA Breast Cancer: a phase III, randomized, open-label study evaluating the efficacy and safety of giredestrant plus the fixed-dose combination of pertuzumab and trastuzumab for subcutaneous injection in patients with previously untreated HER2-positive, estrogen receptor-positive locally advanced or metastatic breast cancer
- PMID: 38789924
- PMCID: PMC11127459
- DOI: 10.1186/s12885-024-12179-9
heredERA Breast Cancer: a phase III, randomized, open-label study evaluating the efficacy and safety of giredestrant plus the fixed-dose combination of pertuzumab and trastuzumab for subcutaneous injection in patients with previously untreated HER2-positive, estrogen receptor-positive locally advanced or metastatic breast cancer
Abstract
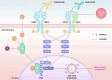
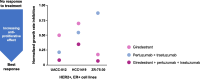
Background: HER2-positive, estrogen receptor-positive breast cancer (HER2+, ER+ BC) is a distinct disease subtype associated with inferior response to chemotherapy plus HER2-targeted therapy compared with HER2+, ER-negative BC. Bi-directional crosstalk leads to cooperation of the HER2 and ER pathways that may drive treatment resistance; thus, simultaneous co-targeting may optimize treatment impact and survival outcomes in patients with HER2+, ER+ BC. First-line (1L) treatment for patients with HER2+ metastatic BC (mBC) is pertuzumab, trastuzumab, and taxane chemotherapy. In clinical practice, dual HER2 blockade plus a fixed number of chemotherapy cycles are given as induction therapy to maximize tumor response, with subsequent HER2-targeted maintenance treatment given as a more tolerable regimen for long-term disease control. For patients whose tumors co-express ER, maintenance endocrine therapy (ET) can be added, but uptake varies due to lack of data from randomized clinical trials investigating the superiority of maintenance ET plus dual HER2 blockade versus dual HER2 blockade alone. Giredestrant, a novel oral selective ER antagonist and degrader, shows promising clinical activity and manageable safety across phase I-II trials of patients with ER+, HER2-negative BC, with therapeutic potential in those with HER2 co-expression.
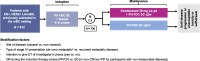
Methods: This phase III, randomized, open-label, two-arm study aims to recruit 812 patients with HER2+, ER+ locally advanced (LA)/mBC into the induction phase (fixed-dose combination of pertuzumab and trastuzumab for subcutaneous injection [PH FDC SC] plus a taxane) to enable 730 patients to be randomized 1:1 to the maintenance phase (giredestrant plus PH FDC SC or PH FDC SC [plus optional ET]), stratified by disease site (visceral versus non-visceral), type of LA/metastatic presentation (de novo versus recurrent), best overall response to induction therapy (partial/complete response versus stable disease), and intent to give ET (yes versus no). The primary endpoint is investigator-assessed progression-free survival. Secondary endpoints include overall survival, objective response rate, clinical benefit rate, duration of response, safety, and patient-reported outcomes.
Discussion: heredERA BC will address whether giredestrant plus dual HER2 blockade is superior to dual HER2 blockade alone, to inform the use of this combination in clinical practice for maintenance 1L treatment of patients with HER2+, ER+ LA/mBC.
Trial registration: ClinicalTrials.gov, NCT05296798; registered on March 25, 2022. Protocol version 3.0 (November 18, 2022).
Sponsor: F. Hoffmann-La Roche Ltd, Grenzacherstrasse 124 4070, Basel, Switzerland.
Keywords: Breast cancer; Estrogen receptor-positive; Giredestrant; HER2-positive; Pertuzumab; Trastuzumab.
© 2024. The Author(s).
Conflict of interest statement
• SK reports a consulting or advisory role with Agendia, Amgen, AstraZeneca, Celgene, Daiichi Sankyo, Genomic Health, Gilead Sciences, Lilly, MSD Oncology, Novartis, Pfizer, pfm medical, F. Hoffmann-La Roche Ltd/Genentech, Inc., Seagen, SOMATEX, Sonoscape, research funding from F. Hoffmann-La Roche Ltd, and reports travel/accommodation/expenses for Daiichi Sankyo, Gilead Sciences, F. Hoffmann-La Roche Ltd.
• CH-W reports honoraria from AstraZeneca, Everything Genetic, Exact Sciences, Gilead Sciences, Lilly, Novartis, Pfizer, F. Hoffmann-La Roche Ltd, Seagen, and research funding from F. Hoffmann-La Roche Ltd.
• YHP reports honoraria from AstraZeneca, Lilly, Merck, Novartis, Pfizer, F. Hoffmann-La Roche Ltd, has a consulting or advisory role with AstraZeneca, Boryung, Daiichi Sankyo, Eisai, Gilead Sciences, Lilly, Menarini, MSD, Novartis, Pfizer, F. Hoffmann-La Roche Ltd, and reports research funding from AstraZeneca, Gencurix (institution), Genome Insight (institution), NGeneBio (institution), Pfizer, F. Hoffmann-La Roche Ltd (institution).
• FF reports research funding from F. Hoffmann-La Roche Ltd.
• MDL reports honoraria from Amgen, AstraZeneca, Gilead Sciences, Ipsen, Lilly, Menarini-Stemline, MSD, Novartis, Pfizer, Pierre Fabre, F. Hoffmann-La Roche Ltd, Sanofi, Seagen, Takeda, a consulting or advisory role with Amgen, AstraZeneca, Gilead Sciences, Ipsen, Lilly, Menarini-Stemline, MSD, Novartis, Pfizer, Pierre Fabre, F. Hoffmann-La Roche Ltd, Sanofi, Seagen, Takeda, a speaker’s bureau with Novartis, Lilly, and research funding from AstraZeneca (institution), Bristol Myers Squibb (institution), Daiichi Sankyo (institution), Eisai (institution), Lilly, Macrogenics (institution), MSD (institution), Novartis (institution), Pfizer (institution), Puma Biotechnology (institution), F. Hoffmann-La Roche Ltd (institution).
• ES-W reports research funding from F. Hoffmann-La Roche Ltd.
• DE reports employment by, and stocks/shares in, F. Hoffmann-La Roche Ltd.
• SH reports employment by Roche Products Limited, stocks/shares in F. Hoffmann-La Roche Ltd, and is a patent holder for PH FDC SC.
• AC reports employment by, and stocks/shares in, F. Hoffmann-La Roche Ltd.
• ÖÖ reports a consulting role with AstraZeneca, Lilly, MSD, Novartis, Pfizer, F. Hoffmann-La Roche Ltd, a Speakers’ Bureau with AstraZeneca, MSD, F. Hoffmann-La Roche Ltd, research funding/grant from BMS, Lilly, MSD, Pfizer, F. Hoffmann-La Roche Ltd, Servier, and medical writing/editorial support from Lilly.
• FM-V reports honoraria from AstraZeneca, Eisai, Gilead Sciences, Lilly, Merck, Novartis, F. Hoffmann-La Roche Ltd, a speakers’ bureau with AstraZeneca, Lilly, research funding from MSD and F. Hoffmann-La Roche Ltd, and travel/accommodation/expenses with AstraZeneca, MSD, F. Hoffmann-La Roche Ltd.
• CM reports employment by Genentech, Inc., and stocks/shares in F. Hoffmann-La Roche Ltd.
• MH reports employment by Genentech, Inc., and stocks/shares in F. Hoffmann-La Roche Ltd.
• ER reports employment by, and stocks/shares in, F. Hoffmann-La Roche Ltd.
• JO’S reports honoraria from AbbVie, Agendia, Amgen, Aptitude Health, AstraZeneca, Bayer, Bristol Myers Squibb, Celgene, Daiichi Sankyo, Eisai, G1 Therapeutics, Genentech, Inc., Gilead Sciences, GRAIL, Halozyme, HERON, Immunomedics, Ipsen, Lilly, Merck, Myriad Pharmaceuticals, Nektar, Novartis, Ontada/McKesson, Pfizer, Pharmacyclics, Pierre Fabre, Prime Oncology, Puma Biotechnology, F. Hoffmann-La Roche Ltd, Samsung, Sanofi, Seagen, Syndax, Synthon, Taiho Oncology, Takeda, a consulting or advisory role with AbbVie, Agendia, Amgen, Aptitude Health, AstraZeneca, Bayer, Bristol Myers Squibb, Celgene, Daiichi Sankyo, Eisai, G1 Therapeutics, Genentech, Gilead Sciences, GRAIL, Halozyme, HERON, Immunomedics, Ipsen, Lilly, Merck, Myriad Pharmaceuticals, Nektar, Novartis, Ontada/McKesson, Pfizer, Pharmacyclics, Pierre Fabre, Prime Oncology, Puma Biotechnology, F. Hoffmann-La Roche Ltd, Samsung, Sanofi, Seagen, Syndax, Synthon, Taiho Oncology, Takeda, a Speakers’ Bureau with AstraZeneca, Lilly, Novartis, Pfizer, Seagen, research funding from Seagen (institution) and F. Hoffmann-La Roche Ltd, and travel/accommodation/expenses with AbbVie, Agendia, Amgen, AstraZeneca, Celgene, Eisai, GRAIL, Ipsen, Lilly, Myriad Pharmaceuticals, Novartis, Pfizer, Puma Biotechnology, F. Hoffmann-La Roche Ltd, Sanofi, Seagen.
• All authors have received research support for third-party writing assistance for this manuscript, furnished by Brian Law, PhD, of Nucleus Global, an Inizio Company, from F. Hoffmann-La Roche Ltd.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

