Enhancing intraneural revascularization following peripheral nerve injury through hypoxic Schwann-cell-derived exosomes: an insight into endothelial glycolysis
- PMID: 38789980
- PMCID: PMC11127458
- DOI: 10.1186/s12951-024-02536-y
Enhancing intraneural revascularization following peripheral nerve injury through hypoxic Schwann-cell-derived exosomes: an insight into endothelial glycolysis
Abstract
Background: Endothelial cell (EC)-driven intraneural revascularization (INRV) and Schwann cells-derived exosomes (SCs-Exos) both play crucial roles in peripheral nerve injury (PNI). However, the interplay between them remains unclear. We aimed to elucidate the effects and underlying mechanisms of SCs-Exos on INRV following PNI.
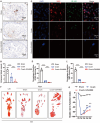
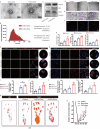
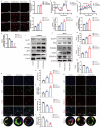
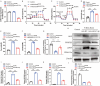
Results: We found that GW4869 inhibited INRV, as well as that normoxic SCs-Exos (N-SCs-Exos) exhibited significant pro-INRV effects in vivo and in vitro that were potentiated by hypoxic SCs-Exos (H-SCs-Exos). Upregulation of glycolysis emerged as a pivotal factor for INRV after PNI, as evidenced by the observation that 3PO administration, a glycolytic inhibitor, inhibited the INRV process in vivo and in vitro. H-SCs-Exos more significantly enhanced extracellular acidification rate/oxygen consumption rate ratio, lactate production, and glycolytic gene expression while simultaneously suppressing acetyl-CoA production and pyruvate dehydrogenase E1 subunit alpha (PDH-E1α) expression than N-SCs-Exos both in vivo and in vitro. Furthermore, we determined that H-SCs-Exos were more enriched with miR-21-5p than N-SCs-Exos. Knockdown of miR-21-5p significantly attenuated the pro-glycolysis and pro-INRV effects of H-SCs-Exos. Mechanistically, miR-21-5p orchestrated EC metabolism in favor of glycolysis by targeting von Hippel-Lindau/hypoxia-inducible factor-1α and PDH-E1α, thereby enhancing hypoxia-inducible factor-1α-mediated glycolysis and inhibiting PDH-E1α-mediated oxidative phosphorylation.
Conclusion: This study unveiled a novel intrinsic mechanism of pro-INRV after PNI, providing a promising therapeutic target for post-injury peripheral nerve regeneration and repair.
Keywords: Exosome; Glycolysis; Intraneural revascularization; Peripheral nerve injury; Schwann cell; Sciatic nerve; miR-21-5p.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Douglas W. Zochodne. Neurobiology of Peripheral nerve regeneration. Cambridge University Press; 2008.
MeSH terms
Substances
Grants and funding
- No.202201020578/Guangzhou Municipal Science and Technology Project
- No.2022A1515012433/Guangdong Basic and Applied Basic Research Foundation
- No.2023WW504/The "five-five" project construction project of the Third Hospital of Sun Yat-sen University
- No.2020001/Sun Yat-Sen University Clinical Research 5010 Program
LinkOut - more resources
Full Text Sources
Medical

