Levels of circulating tumor DNA correlate with tumor volume in gastro-intestinal stromal tumors: an exploratory long-term follow-up study
- PMID: 38790141
- PMCID: PMC11547224
- DOI: 10.1002/1878-0261.13644
Levels of circulating tumor DNA correlate with tumor volume in gastro-intestinal stromal tumors: an exploratory long-term follow-up study
Abstract
Patients with gastro-intestinal stromal tumors (GISTs) undergoing tyrosine kinase inhibitor therapy are monitored with regular computed tomography (CT) scans, exposing patients to cumulative radiation. This exploratory study aimed to evaluate circulating tumor DNA (ctDNA) testing to monitor treatment response and compare changes in ctDNA levels with RECIST 1.1 and total tumor volume measurements. Between 2014 and 2021, six patients with KIT proto-oncogene, receptor tyrosine kinase (KIT) exon-11-mutated GIST from whom long-term plasma samples were collected prospectively were included in the study. ctDNA levels of relevant plasma samples were determined using the KIT exon 11 digital droplet PCR drop-off assay. Tumor volume measurements were performed using a semi-automated approach. In total, 94 of 130 clinically relevant ctDNA samples were analyzed. Upon successful treatment response, ctDNA became undetectable in all patients. At progressive disease, ctDNA was detectable in five out of six patients. Higher levels of ctDNA correlated with larger tumor volumes. Undetectable ctDNA at the time of progressive disease on imaging was consistent with lower tumor volumes compared to those with detectable ctDNA. In summary, ctDNA levels seem to correlate with total tumor volume at the time of progressive disease. Our exploratory study shows promise for including ctDNA testing in treatment follow-up.
Keywords: circulating tumor DNA; follow‐up; gastro‐intestinal stromal tumor; treatment; tumor volume.
© 2024 The Authors. Molecular Oncology published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
Ed Schuuring has received unrestricted grants (all paid to UMCG institution) from Abbott, Biocartis, AstraZeneca, Invitae/Archer, Bayer, Bio‐Rad, Roche, Agena Bioscience, CC Diagnostics, MSD/MERCK, and Boehringer Ingelheim, has received consulting fees (all paid to UMCG institution) from MSD/Merck, AstraZeneca, Roche, Novartis, Bayer, BMS, Lilly, Amgen, Illumina, Agena Bioscience, CC Diagnostics, Janssen Cilag (Johnson & Johnson), Astellas Pharma, GSK, Sinnovisionlab, and Sysmex, has received payments or honoraria (all paid to UMCG institution) from Bio‐Rad, Seracare, Roche, Biocartis, Lilly, Agena Bioscience, and Illumina, has received support for attending meetings and/or travel from Bio‐Rad, Biocartis, Ageno SciencAR, and Illumina, is a board member for the Dutch Society of Pathology (unpaid), European Society of Pathology (unpaid), European Liquid Biopsy Society (unpaid), is a secretary/member of the advisory committee for assessment of molecular diagnostics (cieBOD) (honoraria paid to UMCG institution), is committee member of national guideline advisory (honoraria paid to UMCG institution). Anna K. Reyners has received payments for studies (all paid to UMCG institution) from Deciphera, GSK, MSD, Genmab, Cogent, Tesaro, and Regeneron. Anna K. Reyners is chairperson of the Committee for the Evaluation of Oncological Agents of the Dutch Society of Medical Oncologists (NVMO) and member of the board of the NVMO (paid to the UMCG institution). N Steeghs provided consultation or attended advisory boards for Boehringer Ingelheim, Ellipses Pharma, GlaxoSmithKline, Incyte, Luszana. N Steeghs received research grants from Abbvie, Actuate Therapeutics, Amgen, Array, Ascendis Pharma, AstraZeneca, Bayer, Blueprint Medicines, Boehringer Ingelheim, Bristol‐Myers Squibb, Cantargia, CellCentric, Cogent Biosciences, Cresecendo Biologics, Cytovation, Deciphera, Dragonfly, Eli Lilly, Exelixis, Genentech, GlaxoSmithKline, IDRx, Immunocore, Incyte, InteRNA, Janssen, Kinnate Biopharma, Kling Biotherapeutics, Lixte, Luszana, Merck, Merck Sharp & Dohme, Merus, Molecular Partners, Navire Pharma, Novartis, Numab Therapeutics, Pfizer, Relay Pharmaceuticals, Revolution Medicin, Roche, Sanofi, Seattle Genetics, Taiho, Takeda. All outside the submitted work, all payment to the Netherlands Cancer Institute. Ingrid M.E. Desar received payments for studies (all paid to Radboud University Medical Centre) from Sanofi‐Regeneron. She received a grant of the Dutch Cancer Foundation ‘Koningin Wilhemina Fund’, paid to Radboudumc. She is member of the Dutch Health Care Council, also paid to Radboudumc. Ron H.J. Mathijssen received unrestricted grants (all paid to the institute) for investigator initiated studies from Astellas, Bayer, Boehringer Ingelheim, Cristal Therapeutics, Novartis, Pamgene, Pfizer, Roche, Sanofi and Servier. The other authors declare no conflicts of interest.
Figures

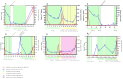
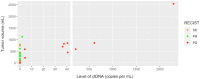
References
-
- Nilsson B, Bümming P, Meis‐Kindblom JM, Odén A, Dortok A, Gustavsson B, et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era‐‐a population‐based study in western Sweden. Cancer. 2005;103:821–829. - PubMed
-
- Søreide K, Sandvik OM, Søreide JA, Giljaca V, Jureckova A, Bulusu VR. Global epidemiology of gastrointestinal stromal tumours (GIST): a systematic review of population‐based cohort studies. Cancer Epidemiol. 2016;40:39–46. - PubMed
-
- Roberts PJ, Eisenberg B. Clinical presentation of gastrointestinal stromal tumors and treatment of operable disease. Eur J Cancer. 2002;38(Suppl 5):S37–S38. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

