The Role of MicroRNAs in HIV Infection
- PMID: 38790203
- PMCID: PMC11120859
- DOI: 10.3390/genes15050574
The Role of MicroRNAs in HIV Infection
Abstract
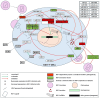
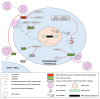
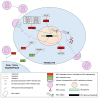
MicroRNAs (miRNAs), a class of small, non-coding RNAs, play a pivotal role in regulating gene expression at the post-transcriptional level. These regulatory molecules are integral to many biological processes and have been implicated in the pathogenesis of various diseases, including Human Immunodeficiency Virus (HIV) infection. This review aims to cover the current understanding of the multifaceted roles miRNAs assume in the context of HIV infection and pathogenesis. The discourse is structured around three primary focal points: (i) elucidation of the mechanisms through which miRNAs regulate HIV replication, encompassing both direct targeting of viral transcripts and indirect modulation of host factors critical for viral replication; (ii) examination of the modulation of miRNA expression by HIV, mediated through either viral proteins or the activation of cellular pathways consequent to viral infection; and (iii) assessment of the impact of miRNAs on the immune response and the progression of disease in HIV-infected individuals. Further, this review delves into the potential utility of miRNAs as biomarkers and therapeutic agents in HIV infection, underscoring the challenges and prospects inherent to this line of inquiry. The synthesis of current evidence positions miRNAs as significant modulators of the host-virus interplay, offering promising avenues for enhancing the diagnosis, treatment, and prevention of HIV infection.
Keywords: HIV; HIV miRNAs; MicroRNA regulation; MicroRNAs; miRNA diagnostics; miRNA therapeutics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Allmer J., Yousef M., editors. miRNomics: MicroRNA Biology and Computational Analysis. Volume 2257. Springer; New York, NY, USA: 2022. Methods in Molecular Biology.
-
- Cantet F., Allmer J., Al Dahouk S., Bonazzi M., Köhler S. RNA-Immunoprecipitation/miRNA-Seq Reveals miRNA-like, Small Noncoding RNAs of Brucella Suis, Translocated into the Cytoplasm of Infected Murine Macrophages; Proceedings of the Brucellosis 2022 International Research Conference; Teramo, Italy. 16–19 September 2022.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

