Comprehensive Bioinformatic Investigation of TP53 Dysregulation in Diverse Cancer Landscapes
- PMID: 38790205
- PMCID: PMC11121236
- DOI: 10.3390/genes15050577
Comprehensive Bioinformatic Investigation of TP53 Dysregulation in Diverse Cancer Landscapes
Abstract
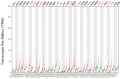
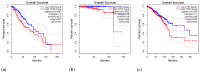
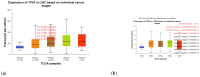
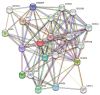
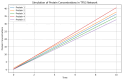
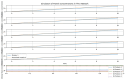
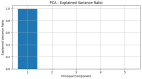
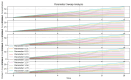
P53 overexpression plays a critical role in cancer pathogenesis by disrupting the intricate regulation of cellular proliferation. Despite its firmly established function as a tumor suppressor, elevated p53 levels can paradoxically contribute to tumorigenesis, influenced by factors such as exposure to carcinogens, genetic mutations, and viral infections. This phenomenon is observed across a spectrum of cancer types, including bladder (BLCA), ovarian (OV), cervical (CESC), cholangiocarcinoma (CHOL), colon adenocarcinoma (COAD), diffuse large B-cell lymphoma (DLBC), esophageal carcinoma (ESCA), head and neck squamous cell carcinoma (HNSC), kidney chromophobe (KICH), kidney renal clear cell carcinoma (KIRC), liver hepatocellular carcinoma (LIHC), lung adenocarcinoma (LUAD), lung squamous cell carcinoma (LUSC), and uterine corpus endometrial carcinoma (UCEC). This broad spectrum of cancers is often associated with increased aggressiveness and recurrence risk. Effective therapeutic strategies targeting tumors with p53 overexpression require a comprehensive approach, integrating targeted interventions aimed at the p53 gene with conventional modalities such as chemotherapy, radiation therapy, and targeted drugs. In this extensive study, we present a detailed analysis shedding light on the multifaceted role of TP53 across various cancers, with a specific emphasis on its impact on disease-free survival (DFS). Leveraging data from the TCGA database and the GTEx dataset, along with GEPIA, UALCAN, and STRING, we identify TP53 overexpression as a significant prognostic indicator, notably pronounced in prostate adenocarcinoma (PRAD). Supported by compelling statistical significance (p < 0.05), our analysis reveals the distinct influence of TP53 overexpression on DFS outcomes in PRAD. Additionally, graphical representations of overall survival (OS) underscore the notable disparity in OS duration between tumors exhibiting elevated TP53 expression (depicted by the red line) and those with lower TP53 levels (indicated by the blue line). The hazard ratio (HR) further emphasizes the profound impact of TP53 on overall survival. Moreover, our investigation delves into the intricate TP53 protein network, unveiling genes exhibiting robust positive correlations with TP53 expression across 13 out of 27 cancers. Remarkably, negative correlations emerge with pivotal tumor suppressor genes. This network analysis elucidates critical proteins, including SIRT1, CBP, p300, ATM, DAXX, HSP 90-alpha, Mdm2, RPA70, 14-3-3 protein sigma, p53, and ASPP2, pivotal in regulating cell cycle dynamics, DNA damage response, and transcriptional regulation. Our study underscores the paramount importance of deciphering TP53 dynamics in cancer, providing invaluable insights into tumor behavior, disease-free survival, and potential therapeutic avenues.
Keywords: TCGA database; TP53 protein network; cancer development; disease-free survival; p53 overexpression; targeted therapy; tumor progression.
Conflict of interest statement
The authors declare that there are no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures












References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

