Real-World Weekly Efficacy Analysis of Faricimab in Patients with Age-Related Macular Degeneration
- PMID: 38790345
- PMCID: PMC11118397
- DOI: 10.3390/bioengineering11050478
Real-World Weekly Efficacy Analysis of Faricimab in Patients with Age-Related Macular Degeneration
Abstract
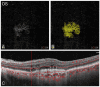
Objectives: This study entailed a weekly analysis of real-world data (RWD) on the safety and efficacy of intravitreal (IVT) faricimab in neovascular age-related macular degeneration (nAMD). Methods: A retrospective, single-centre clinical trial was conducted at the Department of Ophthalmology, University Hospital Zurich, University of Zurich, Switzerland, approved by the Cantonal Ethics Committee of Zurich, Switzerland. Patients with nAMD were included. Data from patient charts and imaging were analysed. The safety and efficacy of the first faricimab injection were evaluated weekly until 4 weeks after injection. Results: Sixty-three eyes with a complete 4-week follow-up were enrolled. Six eyes were treatment-naïve; fifty-seven eyes were switched to faricimab from another treatment. Neither group showed signs of retinal vasculitis during the 4 weeks after injection. Central subfield thickness (CST) and volume (CSV) showed a statistically significant decrease compared to the baseline in the switched group (CST: p = 0.00383; CSV: p = 0.00702) after 4 weeks. The corrected visual acuity returned to the baseline level in both groups. The macular neovascularization area decreased in both groups, but this was not statistically significant. A complete resolution of sub- and intraretinal fluid after 4 weeks was found in 40% (switched) and 75% (naïve) of the treated patients. Conclusions: The weekly follow-ups reflect the structure-function relationship beginning with a fast functional improvement within two weeks after injection followed by a return to near-baseline levels after week 3. The first faricimab injection in our cohort showed a high safety profile and a statistically significant reduction in macular oedema in switched nAMD patients.
Keywords: AMD; CNV; IVI; IVT; MNV; OCT; RWD; age-related macular degeneration; anti-VEGF; exudative; faricimab; intravitreal injection; neovascular; real-world data.
Conflict of interest statement
None of the financial disclosures are relevant to this project.
Figures

References
-
- Michaelson I.C. The mode of development of the vascular system of the retina with some observations on its significance for certain retinal disorders. Trans. Ophthalmol. Soc. UK. 1948:137–180.
-
- Lagrue G., Xheneumont S., Branellec A., Hirbec G., Weil B. A vascular permeability factor elaborated from lymphocytes. I. Demonstration in patients with nephrotic syndrome. Biomedicine. 1975;23:37–40. - PubMed
LinkOut - more resources
Full Text Sources

