Structural and Organizational Strategies of Locomotor Modules during Landing in Patients with Chronic Ankle Instability
- PMID: 38790384
- PMCID: PMC11117571
- DOI: 10.3390/bioengineering11050518
Structural and Organizational Strategies of Locomotor Modules during Landing in Patients with Chronic Ankle Instability
Abstract
Background: Human locomotion involves the coordinated activation of a finite set of modules, known as muscle synergy, which represent the motor control strategy of the central nervous system. However, most prior studies have focused on isolated muscle activation, overlooking the modular organization of motor behavior. Therefore, to enhance comprehension of muscle coordination dynamics during multi-joint movements in chronic ankle instability (CAI), exploring muscle synergies during landing in CAI patients is imperative.
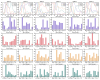
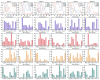
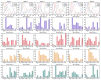
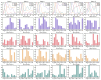
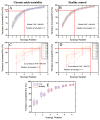
Methods: A total of 22 patients with unilateral CAI and 22 healthy participants were recruited for this research. We employed a recursive model for second-order differential equations to process electromyographic (EMG) data after filtering preprocessing, generating the muscle activation matrix, which was subsequently inputted into the non-negative matrix factorization model for extraction of the muscle synergy. Muscle synergies were classified utilizing the K-means clustering algorithm and Pearson correlation coefficients. Statistical parameter mapping (SPM) was employed for temporal modular parameter analyses.
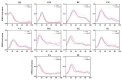
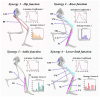
Results: Four muscle synergies were identified in both the CAI and healthy groups. In Synergy 1, only the gluteus maximus showed significantly higher relative weight in CAI compared to healthy controls (p = 0.0035). Synergy 2 showed significantly higher relative weights for the vastus lateralis in the healthy group compared to CAI (p = 0.018), while in Synergy 4, CAI demonstrated significantly higher relative weights of the vastus lateralis compared to healthy controls (p = 0.030). Furthermore, in Synergy 2, the CAI group exhibited higher weights of the tibialis anterior compared to the healthy group (p = 0.042).
Conclusions: The study suggested that patients with CAI exhibit a comparable modular organizational framework to the healthy group. Investigation of amplitude adjustments within the synergy spatial module shed light on the adaptive strategies employed by the tibialis anterior and gluteus maximus muscles to optimize control strategies during landing in patients with CAI. Variances in the muscle-specific weights of the vastus lateralis across movement modules reveal novel biomechanical adaptations in CAI, offering valuable insights for refining rehabilitation protocols.
Keywords: K-means clustering; chronic ankle instability; muscle activation model; muscle synergy; non-negative matrix factorization.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Assessment of Muscle Synergies in Chronic Ankle Instability Patients During Unanticipated and Anticipated Landing.Bioengineering (Basel). 2024 Dec 6;11(12):1237. doi: 10.3390/bioengineering11121237. Bioengineering (Basel). 2024. PMID: 39768055 Free PMC article.
-
Muscle Synergies in People With Chronic Ankle Instability During Anticipated and Unanticipated Landing-Cutting Tasks.J Athl Train. 2023 Feb 1;58(2):143-152. doi: 10.4085/1062-6050-74-21. J Athl Train. 2023. PMID: 34793595 Free PMC article.
-
Muscle mechanics and energetics in chronic ankle instability and copers during landing: Strategies for adaptive adjustments in locomotion pattern.Heliyon. 2025 Jan 11;11(2):e41901. doi: 10.1016/j.heliyon.2025.e41901. eCollection 2025 Jan 30. Heliyon. 2025. PMID: 39897792 Free PMC article.
-
Muscle synergies for evaluating upper limb in clinical applications: A systematic review.Heliyon. 2023 May 11;9(5):e16202. doi: 10.1016/j.heliyon.2023.e16202. eCollection 2023 May. Heliyon. 2023. PMID: 37215841 Free PMC article. Review.
-
Gluteus Medius for Individuals with Chronic Ankle Instability: Assessing Muscle Activity.J Hum Kinet. 2024 Jul 17;94:5-21. doi: 10.5114/jhk/190267. eCollection 2024 Oct. J Hum Kinet. 2024. PMID: 39563768 Free PMC article. Review.
Cited by
-
Adaptive Adjustments in Lower Limb Muscle Coordination during Single-Leg Landing Tasks in Latin Dancers.Biomimetics (Basel). 2024 Aug 13;9(8):489. doi: 10.3390/biomimetics9080489. Biomimetics (Basel). 2024. PMID: 39194468 Free PMC article.
-
Assessment of Muscle Synergies in Chronic Ankle Instability Patients During Unanticipated and Anticipated Landing.Bioengineering (Basel). 2024 Dec 6;11(12):1237. doi: 10.3390/bioengineering11121237. Bioengineering (Basel). 2024. PMID: 39768055 Free PMC article.
-
Analysis of stress response distribution in patients with lateral ankle ligament injuries: a study of neural control strategies utilizing predictive computing models.Front Physiol. 2024 Jul 24;15:1438194. doi: 10.3389/fphys.2024.1438194. eCollection 2024. Front Physiol. 2024. PMID: 39113939 Free PMC article.
References
-
- Gribble P.A., Bleakley C.M., Caulfield B.M., Docherty C.L., Fourchet F., Fong D.T.-P., Hertel J., Hiller C.E., Kaminski T.W., McKeon P.O. Evidence review for the 2016 International Ankle Consortium consensus statement on the prevalence, impact and long-term consequences of lateral ankle sprains. Br. J. Sports Med. 2016;50:1496–1505. doi: 10.1136/bjsports-2016-096189. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

