Abstinence and Fear Experienced during This Period Produce Distinct Cortical and Hippocampal Adaptations in Alcohol-Dependent Rats
- PMID: 38790410
- PMCID: PMC11118749
- DOI: 10.3390/brainsci14050431
Abstinence and Fear Experienced during This Period Produce Distinct Cortical and Hippocampal Adaptations in Alcohol-Dependent Rats
Abstract
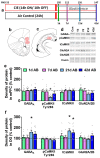
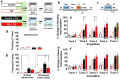
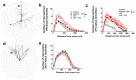
Previous studies demonstrate that ethanol dependence induced by repeating cycles of chronic intermittent ethanol vapor exposure (CIE) followed by protracted abstinence produces significant gray matter damage via myelin dysfunction in the rodent medial prefrontal cortex (mPFC) and alterations in neuronal excitability in the mPFC and the dentate gyrus (DG) of the hippocampus. Specifically, abstinence-induced neuroadaptations have been associated with persistent elevated relapse to drinking. The current study evaluated the effects of forced abstinence for 1 day (d), 7 d, 21 d, and 42 d following seven weeks of CIE on synaptic plasticity proteins in the mPFC and DG. Immunoblotting revealed reduced expression of CaMKII in the mPFC and enhanced expression of GABAA and CaMKII in the DG at the 21 d time point, and the expression of the ratio of GluN2A/2B subunits did not change at any of the time points studied. Furthermore, cognitive performance via Pavlovian trace fear conditioning (TFC) was evaluated in 3 d abstinent rats, as this time point is associated with negative affect. In addition, the expression of the ratio of GluN2A/2B subunits and a 3D structural analysis of neurons in the mPFC and DG were evaluated in 3 d abstinent rats. Behavioral analysis revealed faster acquisition of fear responses and reduced retrieval of fear memories in CIE rats compared to controls. TFC produced hyperplasticity of pyramidal neurons in the mPFC under control conditions and this effect was not evident or blunted in abstinent rats. Neurons in the DG were unaltered. TFC enhanced the GluN2A/2B ratio in the mPFC and reduced the ratio in the DG and was not altered by abstinence. These findings indicate that forced abstinence from CIE produces distinct and divergent alterations in plasticity proteins in the mPFC and DG. Fear learning-induced changes in structural plasticity and proteins contributing to it were more profound in the mPFC during forced abstinence.
Keywords: GluN; Golgi–Cox; alcohol dependence; dentate gyrus; prefrontal cortex; trace fear conditioning.
Conflict of interest statement
The authors declare no competing financial interests in relation to the work described.
Figures

Similar articles
-
Abstinence from ethanol dependence produces concomitant cortical gray matter abnormalities, microstructural deficits and cognitive dysfunction.Eur Neuropsychopharmacol. 2021 Jan;42:22-34. doi: 10.1016/j.euroneuro.2020.11.010. Epub 2020 Dec 2. Eur Neuropsychopharmacol. 2021. PMID: 33279357 Free PMC article.
-
Chronic ethanol exposure differentially alters neuronal function in the medial prefrontal cortex and dentate gyrus.Neuropharmacology. 2021 Mar 1;185:108438. doi: 10.1016/j.neuropharm.2020.108438. Epub 2020 Dec 15. Neuropharmacology. 2021. PMID: 33333103 Free PMC article.
-
Isoxazole-9 reduces enhanced fear responses and retrieval in ethanol-dependent male rats.J Neurosci Res. 2021 Nov;99(11):3047-3065. doi: 10.1002/jnr.24932. Epub 2021 Sep 8. J Neurosci Res. 2021. PMID: 34496069 Free PMC article.
-
Dendritic remodeling of hippocampal neurons is associated with altered NMDA receptor expression in alcohol dependent rats.Mol Cell Neurosci. 2015 Mar;65:153-62. doi: 10.1016/j.mcn.2015.03.008. Epub 2015 Mar 10. Mol Cell Neurosci. 2015. PMID: 25769285 Free PMC article.
-
Learning-induced intrinsic and synaptic plasticity in the rodent medial prefrontal cortex.Neurobiol Learn Mem. 2020 Mar;169:107117. doi: 10.1016/j.nlm.2019.107117. Epub 2019 Nov 23. Neurobiol Learn Mem. 2020. PMID: 31765801 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

