Neuroinflammation and Dyskinesia: A Possible Causative Relationship?
- PMID: 38790492
- PMCID: PMC11118841
- DOI: 10.3390/brainsci14050514
Neuroinflammation and Dyskinesia: A Possible Causative Relationship?
Abstract
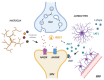
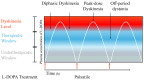
Levodopa (L-DOPA) treatment represents the gold standard therapy for Parkinson's disease (PD) patients. L-DOPA therapy shows many side effects, among them, L-DOPA-induced dyskinesias (LIDs) remain the most problematic. Several are the mechanisms underlying these processes: abnormal corticostriatal neurotransmission, pre- and post-synaptic neuronal events, changes in gene expression, and altered plasticity. In recent years, researchers have also suggested non-neuronal mechanisms as a possible cause for LIDs. We reviewed recent clinical and pre-clinical studies on neuroinflammation contribution to LIDs. Microglia and astrocytes seem to play a strategic role in LIDs phenomenon. In particular, their inflammatory response affects neuron-glia communication, synaptic activity and neuroplasticity, contributing to LIDs development. Finally, we describe possible new therapeutic interventions for dyskinesia prevention targeting glia cells.
Keywords: L-DOPA-induced dyskinesias (LIDs); Parkinson’s disease; levodopa (L-DOPA); neuroinflammation; non-neuronal mechanism.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

