Ca2+-Dependent Cl- Channels in Vascular Tone Regulation during Aging
- PMID: 38791133
- PMCID: PMC11121552
- DOI: 10.3390/ijms25105093
Ca2+-Dependent Cl- Channels in Vascular Tone Regulation during Aging
Abstract
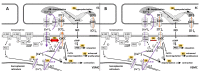
Identifying alterations caused by aging could be an important tool for improving the diagnosis of cardiovascular diseases. Changes in vascular tone regulation involve various mechanisms, like NO synthase activity, activity of the sympathetic nervous system, production of prostaglandin, endothelium-dependent relaxing, and contracting factors, etc. Surprisingly, Ca2+-dependent Cl- channels (CaCCs) are involved in all alterations of the vascular tone regulation mentioned above. Furthermore, we discuss these mechanisms in the context of ontogenetic development and aging. The molecular and electrophysiological mechanisms of CaCCs activation on the cell membrane of the vascular smooth muscle cells (VSMC) and endothelium are explained, as well as the age-dependent changes that imply the activation or inhibition of CaCCs. In conclusion, due to the diverse intracellular concentration of chloride in VSMC and endothelial cells, the activation of CaCCs depends, in part, on intracellular Ca2+ concentration, and, in part, on voltage, leading to fine adjustments of vascular tone. The activation of CaCCs declines during ontogenetic development and aging. This decline in the activation of CaCCs involves a decrease in protein level, the impairment of Ca2+ influx, and probably other alterations in vascular tone regulation.
Keywords: Ca2+-dependent Cl− channels; TMEM16 protein family; aging; phospholipid scramblase; sympathetic nerve activity; vascular tone regulation.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
-
- Seawright J.W., Sreenivasappa H., Gibbs H.C., Padgham S., Shin S.Y., Chaponnier C., Yeh A.T., Trzeciakowski J.P., Woodman C.R., Trache A. Vascular smooth muscle contractile function declines with age in skeletal muscle feed arteries. Front. Physiol. 2018;9:856. doi: 10.3389/fphys.2018.00856. - DOI - PMC - PubMed
-
- Behuliak M., Vavřínová A., Bencze M., Polgárová K., Ergang P., Kuneš J., Vaněčková I., Zicha J. Ontogenetic changes in contribution of calcium sensitization and calcium entry to blood pressure maintenance of Wistar-Kyoto and spontaneously hypertensive rats. J. Hypertens. 2015;33:2443–2454. doi: 10.1097/hjh.0000000000000746. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

