The Puzzle of Aspirin and Iron Deficiency: The Vital Missing Link of the Iron-Chelating Metabolites
- PMID: 38791185
- PMCID: PMC11121054
- DOI: 10.3390/ijms25105150
The Puzzle of Aspirin and Iron Deficiency: The Vital Missing Link of the Iron-Chelating Metabolites
Abstract
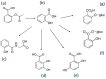
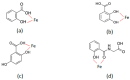
Acetylsalicylic acid or aspirin is the most commonly used drug in the world and is taken daily by millions of people. There is increasing evidence that chronic administration of low-dose aspirin of about 75-100 mg/day can cause iron deficiency anaemia (IDA) in the absence of major gastric bleeding; this is found in a large number of about 20% otherwise healthy elderly (>65 years) individuals. The mechanisms of the cause of IDA in this category of individuals are still largely unknown. Evidence is presented suggesting that a likely cause of IDA in this category of aspirin users is the chelation activity and increased excretion of iron caused by aspirin chelating metabolites (ACMs). It is estimated that 90% of oral aspirin is metabolized into about 70% of the ACMs salicyluric acid, salicylic acid, 2,5-dihydroxybenzoic acid, and 2,3-dihydroxybenzoic acid. All ACMs have a high affinity for binding iron and ability to mobilize iron from different iron pools, causing an overall net increase in iron excretion and altering iron balance. Interestingly, 2,3-dihydroxybenzoic acid has been previously tested in iron-loaded thalassaemia patients, leading to substantial increases in iron excretion. The daily administration of low-dose aspirin for long-term periods is likely to enhance the overall iron excretion in small increments each time due to the combined iron mobilization effect of the ACM. In particular, IDA is likely to occur mainly in populations such as elderly vegetarian adults with meals low in iron content. Furthermore, IDA may be exacerbated by the combinations of ACM with other dietary components, which can prevent iron absorption and enhance iron excretion. Overall, aspirin is acting as a chelating pro-drug similar to dexrazoxane, and the ACM as combination chelation therapy. Iron balance, pharmacological, and other studies on the interaction of iron and aspirin, as well as ACM, are likely to shed more light on the mechanism of IDA. Similar mechanisms of iron chelation through ACM may also be implicated in patient improvements observed in cancer, neurodegenerative, and other disease categories when treated long-term with daily aspirin. In particular, the role of aspirin and ACM in iron metabolism and free radical pathology includes ferroptosis, and may identify other missing links in the therapeutic effects of aspirin in many more diseases. It is suggested that aspirin is the first non-chelating drug described to cause IDA through its ACM metabolites. The therapeutic, pharmacological, toxicological and other implications of aspirin are incomplete without taking into consideration the iron binding and other effects of the ACM.
Keywords: 2,3-dihydroxybenzoic acid; aspirin; drug metabolism; elderly population; genticic acid; iron chelating metabolite; iron deficiency; pharmacology; salicylic acid; salicyluric acid.
Conflict of interest statement
The author declares no conflicts of interest.
Figures
Similar articles
-
New Insights into Aspirin's Anticancer Activity: The Predominant Role of Its Iron-Chelating Antioxidant Metabolites.Antioxidants (Basel). 2024 Dec 29;14(1):29. doi: 10.3390/antiox14010029. Antioxidants (Basel). 2024. PMID: 39857363 Free PMC article. Review.
-
The Importance and Essentiality of Natural and Synthetic Chelators in Medicine: Increased Prospects for the Effective Treatment of Iron Overload and Iron Deficiency.Int J Mol Sci. 2024 Apr 25;25(9):4654. doi: 10.3390/ijms25094654. Int J Mol Sci. 2024. PMID: 38731873 Free PMC article. Review.
-
Chelation studies with 2,3-dihydroxybenzoic acid in patients with beta-thalassaemia major.Br J Haematol. 1976 Aug;33(4):477-85. doi: 10.1111/j.1365-2141.1976.tb03566.x. Br J Haematol. 1976. PMID: 1009020
-
The salicylate metabolite gentisic acid, but not the parent drug, inhibits glucose autoxidation-mediated atherogenic modification of low density lipoprotein.FEBS Lett. 2000 Mar 17;470(1):47-50. doi: 10.1016/s0014-5793(00)01289-8. FEBS Lett. 2000. PMID: 10722843
-
[Metabolic studies on drugs. II. Metabolic products of salicylic, acetylsalicylic, gentisic, and salicyluric acids in rat urine].Hoppe Seylers Z Physiol Chem. 1957 Feb 5;306(4-6):235-46. Hoppe Seylers Z Physiol Chem. 1957. PMID: 13415578 German. No abstract available.
Cited by
-
New Insights into Aspirin's Anticancer Activity: The Predominant Role of Its Iron-Chelating Antioxidant Metabolites.Antioxidants (Basel). 2024 Dec 29;14(1):29. doi: 10.3390/antiox14010029. Antioxidants (Basel). 2024. PMID: 39857363 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

