The Functional Roles of the Src Homology 2 Domain-Containing Inositol 5-Phosphatases SHIP1 and SHIP2 in the Pathogenesis of Human Diseases
- PMID: 38791291
- PMCID: PMC11121230
- DOI: 10.3390/ijms25105254
The Functional Roles of the Src Homology 2 Domain-Containing Inositol 5-Phosphatases SHIP1 and SHIP2 in the Pathogenesis of Human Diseases
Abstract
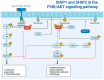
The src homology 2 domain-containing inositol 5-phosphatases SHIP1 and SHIP2 are two proteins involved in intracellular signaling pathways and have been linked to the pathogenesis of several diseases. Both protein paralogs are well known for their involvement in the formation of various kinds of cancer. SHIP1, which is expressed predominantly in hematopoietic cells, has been implicated as a tumor suppressor in leukemogenesis especially in myeloid leukemia, whereas SHIP2, which is expressed ubiquitously, has been implicated as an oncogene in a wider variety of cancer types and is suggested to be involved in the process of metastasis of carcinoma cells. However, there are numerous other diseases, such as inflammatory diseases as well as allergic responses, Alzheimer's disease, and stroke, in which SHIP1 can play a role. Moreover, SHIP2 overexpression was shown to correlate with opsismodysplasia and Alzheimer's disease, as well as metabolic diseases. The SHIP1-inhibitor 3-α-aminocholestane (3AC), and SHIP1-activators, such as AQX-435 and AQX-1125, and SHIP2-inhibitors, such as K161 and AS1949490, have been developed and partly tested in clinical trials, which indicates the importance of the SHIP-paralogs as possible targets in the therapy of those diseases. The aim of this article is to provide an overview of the current knowledge about the involvement of SHIP proteins in the pathogenesis of cancer and other human diseases and to create awareness that SHIP1 and SHIP2 are more than just tumor suppressors and oncogenes.
Keywords: PI3K/AKT signaling pathway; SHIP1; SHIP2; autoimmune diseases; cancer; hematopoietic cancer; human diseases; intracellular signaling; phosphoinositide signaling; therapeutic targets.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

