miRNA Expression Profiles in Isolated Ventricular Cardiomyocytes: Insights into Doxorubicin-Induced Cardiotoxicity
- PMID: 38791311
- PMCID: PMC11121573
- DOI: 10.3390/ijms25105272
miRNA Expression Profiles in Isolated Ventricular Cardiomyocytes: Insights into Doxorubicin-Induced Cardiotoxicity
Abstract
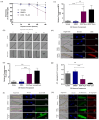
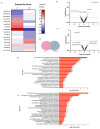
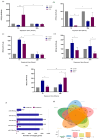
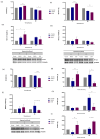
Doxorubicin (DOX), widely used as a chemotherapeutic agent for various cancers, is limited in its clinical utility by its cardiotoxic effects. Despite its widespread use, the precise mechanisms underlying DOX-induced cardiotoxicity at the cellular and molecular levels remain unclear, hindering the development of preventive and early detection strategies. To characterize the cytotoxic effects of DOX on isolated ventricular cardiomyocytes, focusing on the expression of specific microRNAs (miRNAs) and their molecular targets associated with endogenous cardioprotective mechanisms such as the ATP-sensitive potassium channel (KATP), Sirtuin 1 (SIRT1), FOXO1, and GSK3β. We isolated Guinea pig ventricular cardiomyocytes by retrograde perfusion and enzymatic dissociation. We assessed cell morphology, Reactive Oxygen Species (ROS) levels, intracellular calcium, and mitochondrial membrane potential using light microscopy and specific probes. We determined the miRNA expression profile using small RNAseq and validated it using stem-loop qRT-PCR. We quantified mRNA levels of some predicted and validated molecular targets using qRT-PCR and analyzed protein expression using Western blot. Exposure to 10 µM DOX resulted in cardiomyocyte shortening, increased ROS and intracellular calcium levels, mitochondrial membrane potential depolarization, and changes in specific miRNA expression. Additionally, we observed the differential expression of KATP subunits (ABCC9, KCNJ8, and KCNJ11), FOXO1, SIRT1, and GSK3β molecules associated with endogenous cardioprotective mechanisms. Supported by miRNA gene regulatory networks and functional enrichment analysis, these findings suggest that DOX-induced cardiotoxicity disrupts biological processes associated with cardioprotective mechanisms. Further research must clarify their specific molecular changes in DOX-induced cardiac dysfunction and investigate their diagnostic biomarkers and therapeutic potential.
Keywords: FOXO1; GSK3B; KATP; SIRT1; cardioprotection; cardiotoxicity; doxorubicin; miRNAs.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Small Extracellular Vesicles from Breast Cancer Cells Induce Cardiotoxicity.Int J Mol Sci. 2025 Jan 23;26(3):945. doi: 10.3390/ijms26030945. Int J Mol Sci. 2025. PMID: 39940718 Free PMC article.
-
MiR-15b-5p is Involved in Doxorubicin-Induced Cardiotoxicity via Inhibiting Bmpr1a Signal in H9c2 Cardiomyocyte.Cardiovasc Toxicol. 2019 Jun;19(3):264-275. doi: 10.1007/s12012-018-9495-6. Cardiovasc Toxicol. 2019. PMID: 30535663
-
Inhibition of miR-23a attenuates doxorubicin-induced mitochondria-dependent cardiomyocyte apoptosis by targeting the PGC-1α/Drp1 pathway.Toxicol Appl Pharmacol. 2019 Apr 15;369:73-81. doi: 10.1016/j.taap.2019.02.016. Epub 2019 Mar 1. Toxicol Appl Pharmacol. 2019. PMID: 30831132
-
Doxorubicin induced cardio toxicity through sirtuins mediated mitochondrial disruption.Chem Biol Interact. 2022 Sep 25;365:110028. doi: 10.1016/j.cbi.2022.110028. Epub 2022 Jul 31. Chem Biol Interact. 2022. PMID: 35921947 Review.
-
Mitochondrial-Targeted Therapy for Doxorubicin-Induced Cardiotoxicity.Int J Mol Sci. 2022 Feb 9;23(3):1912. doi: 10.3390/ijms23031912. Int J Mol Sci. 2022. PMID: 35163838 Free PMC article. Review.
Cited by
-
Small Extracellular Vesicles from Breast Cancer Cells Induce Cardiotoxicity.Int J Mol Sci. 2025 Jan 23;26(3):945. doi: 10.3390/ijms26030945. Int J Mol Sci. 2025. PMID: 39940718 Free PMC article.
-
Small Molecules Targeting Mitochondria: A Mechanistic Approach to Combating Doxorubicin-Induced Cardiotoxicity.Cardiovasc Toxicol. 2025 Feb;25(2):216-247. doi: 10.1007/s12012-024-09941-7. Epub 2024 Nov 4. Cardiovasc Toxicol. 2025. PMID: 39495464 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

