Chemotherapy-Induced Changes in Plasma Amino Acids and Lipid Oxidation of Resected Patients with Colorectal Cancer: A Background for Future Studies
- PMID: 38791339
- PMCID: PMC11121634
- DOI: 10.3390/ijms25105300
Chemotherapy-Induced Changes in Plasma Amino Acids and Lipid Oxidation of Resected Patients with Colorectal Cancer: A Background for Future Studies
Abstract
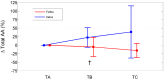
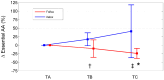
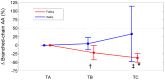
Previous studies have documented that FOLFOX and XELOX therapies negatively impact the metabolism of skeletal muscle and extra-muscle districts. This pilot study tested whether three-month FOLFOX or XELOX therapy produced changes in plasma amino acid levels (PAAL) (an estimation of whole-body amino acid metabolism) and in plasma levels of malondialdehyde (MDA), a marker of lipid hyper oxidation. Fourteen ambulatory, resected patients with colorectal cancer scheduled to receive FOLFOX (n = 9) or XELOX (n = 5) therapy, after overnight fasting, underwent peripheral venous blood sampling, to determine PAAL and MDA before, during, and at the end of three-month therapy. Fifteen healthy matched subjects (controls) only underwent measures of PAAL at baseline. The results showed changes in 87.5% of plasma essential amino acids (EAAs) and 38.4% of non-EAAs in patients treated with FOLFOX or XELOX. These changes in EAAs occurred in two opposite directions: EAAs decreased with FOLFOX and increased or did not decrease with XELOX (interactions: from p = 0.034 to p = 0.003). Baseline plasma MDA levels in both FOLFOX and XELOX patients were above the normal range of values, and increased, albeit not significantly, during therapy. In conclusion, three-month FOLFOX or XELOX therapy affected plasma EAAs differently but not the baseline MDA levels, which were already high.
Keywords: FOLFOX and XELOX therapy; colorectal cancer; plasma amino acid changes; plasma malondialdehyde changes; potential clinical implications; primary tumor location.
Conflict of interest statement
This study received funding (fee for publication) from Professional Dietetics S.p.A., Milano, Italy. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication. No author declares any other competing interests, except for R.A. R.A. who is the scientific consultant of Professional Dietetics, Milano, Italy.
Figures


Similar articles
-
Peripheral neurotoxicity of oxaliplatin in combination with 5-fluorouracil (FOLFOX) or capecitabine (XELOX): a prospective evaluation of 150 colorectal cancer patients.Ann Oncol. 2012 Dec;23(12):3116-3122. doi: 10.1093/annonc/mds208. Epub 2012 Aug 2. Ann Oncol. 2012. PMID: 22865779 Clinical Trial.
-
Oxaliplatin-Fluoropyrimidine Combination (XELOX) Therapy Does Not Affect Plasma Amino Acid Levels and Plasma Markers of Oxidative Stress in Colorectal Cancer Surgery Patients: A Pilot Study.Nutrients. 2019 Nov 5;11(11):2667. doi: 10.3390/nu11112667. Nutrients. 2019. PMID: 31694176 Free PMC article.
-
Capecitabine plus oxaliplatin (XELOX) versus 5-fluorouracil/folinic acid plus oxaliplatin (FOLFOX-4) as second-line therapy in metastatic colorectal cancer: a randomized phase III noninferiority study.Ann Oncol. 2008 Oct;19(10):1720-6. doi: 10.1093/annonc/mdn370. Epub 2008 Jun 10. Ann Oncol. 2008. PMID: 18550577 Clinical Trial.
-
XELOX vs. FOLFOX in metastatic colorectal cancer: An updated meta-analysis.Cancer Invest. 2016;34(2):94-104. doi: 10.3109/07357907.2015.1104689. Epub 2016 Feb 11. Cancer Invest. 2016. PMID: 26864862 Review.
-
FOLFOX plus anti-epidermal growth factor receptor (EGFR) monoclonal antibody (mAb) is an effective first-line treatment for patients with RAS-wild left-sided metastatic colorectal cancer: A meta-analysis.Medicine (Baltimore). 2018 Mar;97(10):e0097. doi: 10.1097/MD.0000000000010097. Medicine (Baltimore). 2018. PMID: 29517682 Free PMC article. Review.
References
-
- Argilés G., Tabernero J., Labianca R., Hochhauser D., Salazar R., Iveson T., Laurent-Puig P., Quirke P., Yoshino T., Taieb J., et al. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020;31:1291–1305. doi: 10.1016/j.annonc.2020.06.022. - DOI - PubMed
-
- Mitani S., Kadowaki S., Komori A., Sugiyama K., Narita Y., Taniguchi H., Ura T., Ando M., Sato Y., Yamaura H., et al. Acute hyperammonemic encephalopathy after fluoropyrimidine-based chemotherapy: A case series and review of the literature. Medicine. 2017;96:e6874. doi: 10.1097/MD.0000000000006874. - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

