Mechanism Analysis of Antimicrobial Peptide NoPv1 Related to Potato Late Blight through a Computer-Aided Study
- PMID: 38791351
- PMCID: PMC11121460
- DOI: 10.3390/ijms25105312
Mechanism Analysis of Antimicrobial Peptide NoPv1 Related to Potato Late Blight through a Computer-Aided Study
Abstract
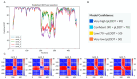
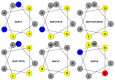
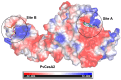
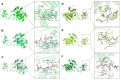
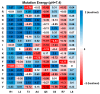
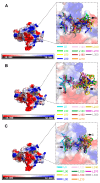
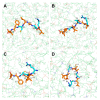
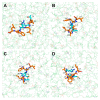
Phytophthora infestans (Mont.) de Bary, the oomycotic pathogen responsible for potato late blight, is the most devastating disease of potato production. The primary pesticides used to control oomycosis are phenyl amide fungicides, which cause environmental pollution and toxic residues harmful to both human and animal health. To address this, an antimicrobial peptide, NoPv1, has been screened to target Plasmopara viticola cellulose synthase 2 (PvCesA2) to inhibit the growth of Phytophthora infestans (P. infestans). In this study, we employed AlphaFold2 to predict the three-dimensional structure of PvCesA2 along with NoPv peptides. Subsequently, utilizing computational methods, we dissected the interaction mechanism between PvCesA2 and these peptides. Based on this analysis, we performed a saturation mutation of NoPv1 and successfully obtained the double mutants DP1 and DP2 with a higher affinity for PvCesA2. Meanwhile, dynamics simulations revealed that both DP1 and DP2 utilize a mechanism akin to the barrel-stave model for penetrating the cell membrane. Furthermore, the predicted results showed that the antimicrobial activity of DP1 was superior to that of NoPv1 without being toxic to human cells. These findings may offer insights for advancing the development of eco-friendly pesticides targeting various oomycete diseases, including late blight.
Keywords: Phytophthora infestans; biopesticide; dynamic simulation; molecular docking; potato.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
NoPv1: a synthetic antimicrobial peptide aptamer targeting the causal agents of grapevine downy mildew and potato late blight.Sci Rep. 2020 Oct 16;10(1):17574. doi: 10.1038/s41598-020-73027-x. Sci Rep. 2020. PMID: 33067553 Free PMC article.
-
Endophytic Bacillus subtilis H17-16 effectively inhibits Phytophthora infestans, the pathogen of potato late blight, and its potential application.Pest Manag Sci. 2023 Dec;79(12):5073-5086. doi: 10.1002/ps.7717. Epub 2023 Aug 23. Pest Manag Sci. 2023. PMID: 37572366
-
Improved Genome Sequence and Gene Annotation Resource for the Potato Late Blight Pathogen Phytophthora infestans.Mol Plant Microbe Interact. 2020 Aug;33(8):1025-1028. doi: 10.1094/MPMI-02-20-0023-A. Epub 2020 Jun 10. Mol Plant Microbe Interact. 2020. PMID: 32310703
-
Genetic factors encoding resistance to late blight caused by Phytophthora infestans (Mont.) de Bary on the potato genetic map.Cell Mol Biol Lett. 2004;9(4B):855-67. Cell Mol Biol Lett. 2004. PMID: 15647802 Review.
-
How Does Phytophthora infestans Evade Control Efforts? Modern Insight Into the Late Blight Disease.Phytopathology. 2018 Aug;108(8):916-924. doi: 10.1094/PHYTO-04-18-0130-IA. Epub 2018 Jul 6. Phytopathology. 2018. PMID: 29979126 Review.
References
-
- Naumann M., Koch M., Thiel H., Gransee A., Pawelzik E. The Importance of Nutrient Management for Potato Production Part II: Plant Nutrition and Tuber Quality. Potato Res. 2019;63:121–137. doi: 10.1007/s11540-019-09430-3. - DOI
-
- Austin Bourke P.M. Emergence of Potato Blight, 1843–1846. Nature. 1964;203:805–808. doi: 10.1038/203805a0. - DOI
-
- Yuen J. Pathogens which threaten food security: Phytophthora infestans, the potato late blight pathogen. Food Secur. 2021;13:247–253. doi: 10.1007/s12571-021-01141-3. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

