CXCL8 Knockout: A Key to Resisting Pasteurella multocida Toxin-Induced Cytotoxicity
- PMID: 38791369
- PMCID: PMC11121343
- DOI: 10.3390/ijms25105330
CXCL8 Knockout: A Key to Resisting Pasteurella multocida Toxin-Induced Cytotoxicity
Abstract
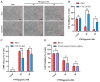
Pasteurella multocida, a zoonotic pathogen that produces a 146-kDa modular toxin (PMT), causes progressive atrophic rhinitis with severe turbinate bone degradation in pigs. However, its mechanism of cytotoxicity remains unclear. In this study, we expressed PMT, purified it in a prokaryotic expression system, and found that it killed PK15 cells. The host factor CXCL8 was significantly upregulated among the differentially expressed genes in a transcriptome sequencing analysis and qPCR verification. We constructed a CXCL8-knockout cell line with a CRISPR/Cas9 system and found that CXCL8 knockout significantly increased resistance to PMT-induced cell apoptosis. CXCL8 knockout impaired the cleavage efficiency of apoptosis-related proteins, including Caspase3, Caspase8, and PARP1, as demonstrated with Western blot. In conclusion, these findings establish that CXCL8 facilitates PMT-induced PK15 cell death, which involves apoptotic pathways; this observation documents that CXCL8 plays a key role in PMT-induced PK15 cell death.
Keywords: CXCL8; PK15 cells; Pasteurella multocida toxin; apoptosis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

