Electro-Metabolic Coupling of Cumulus-Oocyte Complex
- PMID: 38791387
- PMCID: PMC11120766
- DOI: 10.3390/ijms25105349
Electro-Metabolic Coupling of Cumulus-Oocyte Complex
Abstract
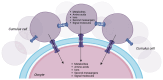
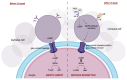
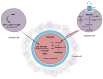
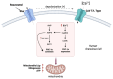
Oocyte-cumulus cell interaction is essential for oocyte maturation and competence. The bidirectional crosstalk network mediated by gap junctions is fundamental for the metabolic cooperation between these cells. As cumulus cells exhibit a more glycolytic phenotype, they can provide metabolic substrates that the oocyte can use to produce ATP via oxidative phosphorylation. The impairment of mitochondrial activity plays a crucial role in ovarian aging and, thus, in fertility, determining the success or failure of assisted reproductive techniques. This review aims to deepen the knowledge about the electro-metabolic coupling of the cumulus-oocyte complex and to hypothesize a putative role of potassium channel modulators in order to improve fertility, promote intracellular Ca2+ influx, and increase the mitochondrial biogenesis and resulting ATP levels in cumulus cells.
Keywords: cumulus cells; gap junctions; glucose metabolism; oocyte; resveratrol.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

