Mesothelioma-Associated Fibroblasts Modulate the Response of Mesothelioma Patient-Derived Organoids to Chemotherapy via Interleukin-6
- PMID: 38791392
- PMCID: PMC11121414
- DOI: 10.3390/ijms25105355
Mesothelioma-Associated Fibroblasts Modulate the Response of Mesothelioma Patient-Derived Organoids to Chemotherapy via Interleukin-6
Abstract
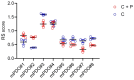
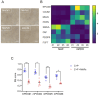
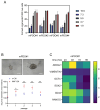
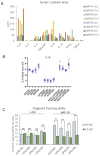
Malignant pleural mesothelioma (MPM) remains an incurable disease. This is partly due to the lack of experimental models that fully recapitulate the complexity and heterogeneity of MPM, a major challenge for therapeutic management of the disease. In addition, the contribution of the MPM microenvironment is relevant for the adaptive response to therapy. We established mesothelioma patient-derived organoid (mPDO) cultures from MPM pleural effusions and tested their response to pemetrexed and cisplatin. We aimed to evaluate the contribution of mesothelioma-associated fibroblasts (MAFs) to the response to pemetrexed and cisplatin (P+C). Organoid cultures were obtained from eight MPM patients using specific growth media and conditions to expand pleural effusion-derived cells. Flow cytometry was used to verify the similarity of the organoid cultures to the original samples. MAFs were isolated and co-cultured with mPDOs, and the addition of MAFs reduced the sensitivity of mPDOs to P+C. Organoid formation and expression of cancer stem cell markers such as ABCG2, NANOG, and CD44 were altered by conditioned media from treated MAFs. We identified IL-6 as the major contributor to the attenuated response to chemotherapy. IL-6 secretion by MAFs is correlated with increased resistance of mPDOs to pemetrexed and cisplatin.
Keywords: IL-6; PDO; chemoresistance; cisplatin; cocultures; mesothelioma patient-derived organoids; mesothelioma-associated fibroblasts; pemetrexed.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Baas P., Daumont M.J., Lacoin L., Penrod J.R., Carroll R., Venkatesan S., Ubhi H., Calleja A., Snee M. Treatment patterns and outcomes for patients with malignant pleural mesothelioma in England in 2013-2017: A nationwide CAS registry analysis from the I-O Optimise initiative. Lung Cancer. 2021;162:185–193. doi: 10.1016/j.lungcan.2021.11.001. - DOI - PubMed
-
- Meirson T., Pentimalli F., Cerza F., Baglio G., Gray S.G., Correale P., Krstic-Demonacos M., Markel G., Giordano A., Bomze D., et al. Comparison of 3 Randomized Clinical Trials of Frontline Therapies for Malignant Pleural Mesothelioma. JAMA Netw. Open. 2022;5:e221490. doi: 10.1001/jamanetworkopen.2022.1490. - DOI - PMC - PubMed
-
- Fennell D.A., Ewings S., Ottensmeier C., Califano R., Hanna G.G., Hill K., Danson S., Steele N., Nye M., Johnson L., et al. Nivolumab versus placebo in patients with relapsed malignant mesothelioma (CONFIRM): A multicentre, double-blind, randomised, phase 3 trial. Lancet Oncol. 2021;22:1530–1540. doi: 10.1016/S1470-2045(21)00471-X. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

