Protopine and Allocryptopine Interactions with Plasma Proteins
- PMID: 38791436
- PMCID: PMC11121924
- DOI: 10.3390/ijms25105398
Protopine and Allocryptopine Interactions with Plasma Proteins
Abstract
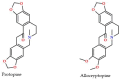
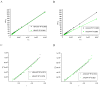
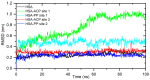
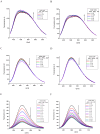
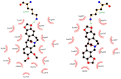
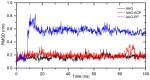
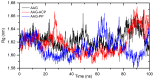
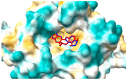
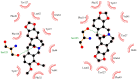
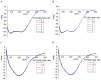
A comprehensive study of the interactions of human serum albumin (HSA) and α-1-acid glycoprotein (AAG) with two isoquinoline alkaloids, i.e., allocryptopine (ACP) and protopine (PP), was performed. The UV-Vis spectroscopy, molecular docking, competitive binding assays, and circular dichroism (CD) spectroscopy were used for the investigations. The results showed that ACP and PP form spontaneous and stable complexes with HSA and AAG, with ACP displaying a stronger affinity towards both proteins. Molecular docking studies revealed the preferential binding of ACP and PP to specific sites within HSA, with site 2 (IIIA) being identified as the favored location for both alkaloids. This was supported by competitive binding assays using markers specific to HSA's drug binding sites. Similarly, for AAG, a decrease in fluorescence intensity upon addition of the alkaloids to AAG/quinaldine red (QR) complexes indicated the replacement of the marker by the alkaloids, with ACP showing a greater extent of replacement than PP. CD spectroscopy showed that the proteins' structures remained largely unchanged, suggesting that the formation of complexes did not significantly perturb the overall spatial configuration of these macromolecules. These findings are crucial for advancing the knowledge on the natural product-protein interactions and the future design of isoquinoline alkaloid-based therapeutics.
Keywords: albumin; allocryptopine; orosomucoid; protopine; spectroscopy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Zielińska S., Jezierska-Domaradzka A., Wójciak-Kosior M., Sowa I., Junka A., Matkowski A.M. Greater Celandine’s ups and downs−21 centuries of medicinal uses of Chelidonium majus from the viewpoint of today’s pharmacology. Front. Pharmacol. 2018;9:299. doi: 10.3389/fphar.2018.00299. - DOI - PMC - PubMed
-
- Cahlikova L., Kavano I., Rezacova M., Blunden G., Hulcova D., Havelek R. The Amaryllidaceae alkaloids haemanthamine, haemanthidine and their semisynthetic derivatives as potential drugs. Phytochem. Rev. 2021;20:303–323. doi: 10.1007/s11101-020-09675-8. - DOI
-
- Mathew B., Parambi D.G.T., Singh M., Hendawy O.M., Al-Sanea M.M., Bakr R.B. Naturally Occurring Chemicals against Alzheimer’s Disease. Elsevier; Amsterdam, The Netherlands: 2021. pp. 167–174. Chapter 3.1.12—Protopine. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

