Presenilin Deficiency Results in Cellular Cholesterol Accumulation by Impairment of Protein Glycosylation and NPC1 Function
- PMID: 38791456
- PMCID: PMC11121565
- DOI: 10.3390/ijms25105417
Presenilin Deficiency Results in Cellular Cholesterol Accumulation by Impairment of Protein Glycosylation and NPC1 Function
Abstract
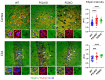
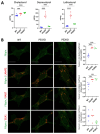
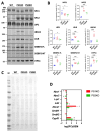
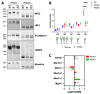
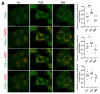
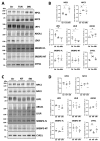
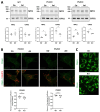
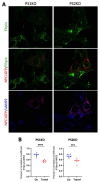
Presenilin proteins (PS1 and PS2) represent the catalytic subunit of γ-secretase and play a critical role in the generation of the amyloid β (Aβ) peptide and the pathogenesis of Alzheimer disease (AD). However, PS proteins also exert multiple functions beyond Aβ generation. In this study, we examine the individual roles of PS1 and PS2 in cellular cholesterol metabolism. Deletion of PS1 or PS2 in mouse models led to cholesterol accumulation in cerebral neurons. Cholesterol accumulation was also observed in the lysosomes of embryonic fibroblasts from Psen1-knockout (PS1-KO) and Psen2-KO (PS2-KO) mice and was associated with decreased expression of the Niemann-Pick type C1 (NPC1) protein involved in intracellular cholesterol transport in late endosomal/lysosomal compartments. Mass spectrometry and complementary biochemical analyses also revealed abnormal N-glycosylation of NPC1 and several other membrane proteins in PS1-KO and PS2-KO cells. Interestingly, pharmacological inhibition of N-glycosylation resulted in intracellular cholesterol accumulation prominently in lysosomes and decreased NPC1, thereby resembling the changes in PS1-KO and PS2-KO cells. In turn, treatment of PS1-KO and PS2-KO mouse embryonic fibroblasts (MEFs) with the chaperone inducer arimoclomol partially normalized NPC1 expression and rescued lysosomal cholesterol accumulation. Additionally, the intracellular cholesterol accumulation in PS1-KO and PS2-KO MEFs was prevented by overexpression of NPC1. Collectively, these data indicate that a loss of PS function results in impaired protein N-glycosylation, which eventually causes decreased expression of NPC1 and intracellular cholesterol accumulation. This mechanism could contribute to the neurodegeneration observed in PS KO mice and potentially to the pathogenesis of AD.
Keywords: N-glycosylation; NPC1; Presenilin; cholesterol; lysosome.
Conflict of interest statement
J.S. has financial interests in iNeuro Therapeutics and Paros Bio, both in the early stages of developing therapies for Alzheimer’s disease. J.S.’s interests are managed by Mass General Brigham in accordance with the institutional conflict of interest policies.
Figures

References
-
- De Strooper B., Annaert W., Cupers P., Saftig P., Craessaerts K., Mumm J.S., Schroeter E.H., Schrijvers V., Wolfe M.S., Ray W.J., et al. A Presenilin-1-Dependent Gamma-Secretase-like Protease Mediates Release of Notch Intracellular Domain. Nature. 1999;398:518–522. doi: 10.1038/19083. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

