Current Knowledge and Perspectives of Phage Therapy for Combating Refractory Wound Infections
- PMID: 38791502
- PMCID: PMC11122179
- DOI: 10.3390/ijms25105465
Current Knowledge and Perspectives of Phage Therapy for Combating Refractory Wound Infections
Abstract
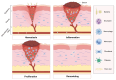
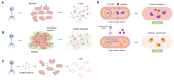
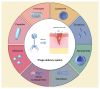
Wound infection is one of the most important factors affecting wound healing, so its effective control is critical to promote the process of wound healing. However, with the increasing prevalence of multi-drug-resistant (MDR) bacterial strains, the prevention and treatment of wound infections are now more challenging, imposing heavy medical and financial burdens on patients. Furthermore, the diminishing effectiveness of conventional antimicrobials and the declining research on new antibiotics necessitate the urgent exploration of alternative treatments for wound infections. Recently, phage therapy has been revitalized as a promising strategy to address the challenges posed by bacterial infections in the era of antibiotic resistance. The use of phage therapy in treating infectious diseases has demonstrated positive results. This review provides an overview of the mechanisms, characteristics, and delivery methods of phage therapy for combating pathogenic bacteria. Then, we focus on the clinical application of various phage therapies in managing refractory wound infections, such as diabetic foot infections, as well as traumatic, surgical, and burn wound infections. Additionally, an analysis of the potential obstacles and challenges of phage therapy in clinical practice is presented, along with corresponding strategies for addressing these issues. This review serves to enhance our understanding of phage therapy and provides innovative avenues for addressing refractory infections in wound healing.
Keywords: MDR; alternative treatments; antibiotic resistance; phage therapy; wound healing; wound infection.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

