A Brain Anti-Senescence Transcriptional Program Triggered by Hypothalamic-Derived Exosomal microRNAs
- PMID: 38791505
- PMCID: PMC11122052
- DOI: 10.3390/ijms25105467
A Brain Anti-Senescence Transcriptional Program Triggered by Hypothalamic-Derived Exosomal microRNAs
Abstract
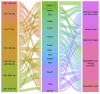
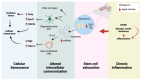
In contrast to the hypothesis that aging results from cell-autonomous deterioration processes, the programmed longevity theory proposes that aging arises from a partial inactivation of a "longevity program" aimed at maintaining youthfulness in organisms. Supporting this hypothesis, age-related changes in organisms can be reversed by factors circulating in young blood. Concordantly, the endocrine secretion of exosomal microRNAs (miRNAs) by hypothalamic neural stem cells (htNSCs) regulates the aging rate by enhancing physiological fitness in young animals. However, the specific molecular mechanisms through which hypothalamic-derived miRNAs exert their anti-aging effects remain unexplored. Using experimentally validated miRNA-target gene interactions and single-cell transcriptomic data of brain cells during aging and heterochronic parabiosis, we identify the main pathways controlled by these miRNAs and the cell-type-specific gene networks that are altered due to age-related loss of htNSCs and the subsequent decline in specific miRNA levels in the cerebrospinal fluid (CSF). Our bioinformatics analysis suggests that these miRNAs modulate pathways associated with senescence and cellular stress response, targeting crucial genes such as Cdkn2a, Rps27, and Txnip. The oligodendrocyte lineage appears to be the most responsive to age-dependent loss of exosomal miRNA, leading to significant derepression of several miRNA target genes. Furthermore, heterochronic parabiosis can reverse age-related upregulation of specific miRNA-targeted genes, predominantly in brain endothelial cells, including senescence promoting genes such as Cdkn1a and Btg2. Our findings support the presence of an anti-senescence mechanism triggered by the endocrine secretion of htNSC-derived exosomal miRNAs, which is associated with a youthful transcriptional signature.
Keywords: aging; bioinformatics; exosomes; miRNAs; neural stem cells; transcriptomics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Intracellular and exosomal microRNAome profiling of human vascular smooth muscle cells during replicative senescence.Am J Physiol Heart Circ Physiol. 2021 Oct 1;321(4):H770-H783. doi: 10.1152/ajpheart.00058.2021. Epub 2021 Sep 10. Am J Physiol Heart Circ Physiol. 2021. PMID: 34506226
-
Hypothalamic stem cells control ageing speed partly through exosomal miRNAs.Nature. 2017 Aug 3;548(7665):52-57. doi: 10.1038/nature23282. Epub 2017 Jul 26. Nature. 2017. PMID: 28746310 Free PMC article.
-
Heterochronic parabiosis reprograms the mouse brain transcriptome by shifting aging signatures in multiple cell types.Nat Aging. 2023 Mar;3(3):327-345. doi: 10.1038/s43587-023-00373-6. Epub 2023 Mar 9. Nat Aging. 2023. PMID: 37118429 Free PMC article.
-
The role of exosomes and microRNAs in senescence and aging.Adv Drug Deliv Rev. 2013 Mar;65(3):368-75. doi: 10.1016/j.addr.2012.07.010. Epub 2012 Jul 20. Adv Drug Deliv Rev. 2013. PMID: 22820533 Review.
-
Emerging role of stem cell-derived extracellular microRNAs in age-associated human diseases and in different therapies of longevity.Ageing Res Rev. 2020 Jan;57:100979. doi: 10.1016/j.arr.2019.100979. Epub 2019 Nov 5. Ageing Res Rev. 2020. PMID: 31704472 Review.
Cited by
-
Therapeutic Efficacy and Promise of Human Umbilical Cord Mesenchymal Stem Cell-Derived Extracellular Vesicles in Aging and Age-Related Disorders.Int J Mol Sci. 2024 Dec 30;26(1):225. doi: 10.3390/ijms26010225. Int J Mol Sci. 2024. PMID: 39796081 Free PMC article. Review.
-
Impacts of systemic milieu on cerebrovascular and brain aging: insights from heterochronic parabiosis, blood exchange, and plasma transfer experiments.Geroscience. 2025 May 23. doi: 10.1007/s11357-025-01657-y. Online ahead of print. Geroscience. 2025. PMID: 40407975 Review.
-
An optimized method for directed differentiation of hypothalamic neural stem cells in a 3D culture system.Sci Rep. 2025 May 27;15(1):18542. doi: 10.1038/s41598-025-02847-6. Sci Rep. 2025. PMID: 40425660 Free PMC article.
-
Age-Related sncRNAs in Human Hippocampal Tissue Samples: Focusing on Deregulated miRNAs.Int J Mol Sci. 2024 Nov 29;25(23):12872. doi: 10.3390/ijms252312872. Int J Mol Sci. 2024. PMID: 39684581 Free PMC article.
-
Epigenetics-targeted drugs: current paradigms and future challenges.Signal Transduct Target Ther. 2024 Nov 26;9(1):332. doi: 10.1038/s41392-024-02039-0. Signal Transduct Target Ther. 2024. PMID: 39592582 Free PMC article. Review.
References
-
- Eichhorn S.W., Guo H., McGeary S.E., Rodriguez-Mias R.A., Shin C., Baek D., Hsu S.H., Ghoshal K., Villen J., Bartel D.P. mRNA destabilization is the dominant effect of mammalian microRNAs by the time substantial repression ensues. Mol. Cell. 2014;56:104–115. doi: 10.1016/j.molcel.2014.08.028. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

