Molecular Characterization and Therapeutic Opportunities in KRAS Wildtype Pancreatic Ductal Adenocarcinoma
- PMID: 38791940
- PMCID: PMC11119482
- DOI: 10.3390/cancers16101861
Molecular Characterization and Therapeutic Opportunities in KRAS Wildtype Pancreatic Ductal Adenocarcinoma
Abstract
Purpose: To investigate the molecular characteristics of and potential for precision medicine in KRAS wildtype pancreatic ductal adenocarcinoma (PDAC).
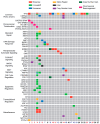
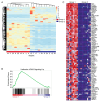
Patients and methods: We investigated 27 patients with KRASWT PDAC at our institution. Clinical data were obtained via chart review. Tumor specimens for each subject were interrogated for somatic single nucleotide variants, insertion and deletions, and copy number variants by DNA sequencing. Gene fusions were detected from RNA-seq. A patient-derived organoid (PDO) was developed from a patient with a MET translocation and expanded ex vivo to predict therapeutic sensitivity prior to enrollment in a phase 2 clinical trial.
Results: Transcriptomic analysis showed our cohort may be stratified by the relative gene expression of the KRAS signaling cascade. The PDO derived from our patient harboring a TFG-MET rearrangement was found to have in vitro sensitivity to the multi-tyrosine kinase inhibitor crizotinib. The patient was enrolled in the phase 2 SPARTA clinical trial and received monotherapy with vebrelitinib, a c-MET inhibitor, and achieved a partial and durable response.
Conclusions: KRASWT PDAC is molecularly distinct from KRASMUT and enriched with potentially actionable genetic variants. In our study, transcriptomic profiling revealed that the KRAS signaling cascade may play a key role in KRASWT PDAC. Our report of a KRASWT PDAC patient with TFG-MET rearrangement who responded to a cMET inhibitor further supports the pursuit of precision oncology in this sub-population. Identification of targetable mutations, perhaps through approaches like RNA-seq, can help enable precision-driven approaches to select optimal treatment based on tumor characteristics.
Keywords: APL-101; KRAS wildtype; MET fusion; PLB1001; pancreatic ductal adenocarcinoma; vebreltinib.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous

