Update on Renal Cell Carcinoma Diagnosis with Novel Imaging Approaches
- PMID: 38792005
- PMCID: PMC11120239
- DOI: 10.3390/cancers16101926
Update on Renal Cell Carcinoma Diagnosis with Novel Imaging Approaches
Abstract
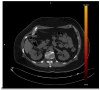
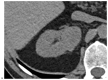
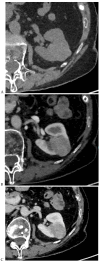
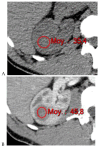
This review highlights recent advances in renal cell carcinoma (RCC) imaging. It begins with dual-energy computed tomography (DECT), which has demonstrated a high diagnostic accuracy in the evaluation of renal masses. Several studies have suggested the potential benefits of iodine quantification, particularly for distinguishing low-attenuation, true enhancing solid masses from hyperdense cysts. By determining whether or not a renal mass is present, DECT could avoid the need for additional imaging studies, thereby reducing healthcare costs. DECT can also provide virtual unenhanced images, helping to reduce radiation exposure. The review then provides an update focusing on the advantages of multiparametric magnetic resonance (MR) imaging performance in the histological subtyping of RCC and in the differentiation of benign from malignant renal masses. A proposed standardized stepwise reading of images helps to identify clear cell RCC and papillary RCC with a high accuracy. Contrast-enhanced ultrasound may represent a promising diagnostic tool for the characterization of solid and cystic renal masses. Several combined pharmaceutical imaging strategies using both sestamibi and PSMA offer new opportunities in the diagnosis and staging of RCC, but their role in risk stratification needs to be evaluated. Although radiomics and tumor texture analysis are hampered by poor reproducibility and need standardization, they show promise in identifying new biomarkers for predicting tumor histology, clinical outcomes, overall survival, and the response to therapy. They have a wide range of potential applications but are still in the research phase. Artificial intelligence (AI) has shown encouraging results in tumor classification, grade, and prognosis. It is expected to play an important role in assessing the treatment response and advancing personalized medicine. The review then focuses on recently updated algorithms and guidelines. The Bosniak classification version 2019 incorporates MRI, precisely defines previously vague imaging terms, and allows a greater proportion of masses to be placed in lower-risk classes. Recent studies have reported an improved specificity of the higher-risk categories and better inter-reader agreement. The clear cell likelihood score, which adds standardization to the characterization of solid renal masses on MRI, has been validated in recent studies with high interobserver agreement. Finally, the review discusses the key imaging implications of the 2017 AUA guidelines for renal masses and localized renal cancer.
Keywords: AUA guidelines; Bosniak classification version 2019; PSMA PET/CT; artificial intelligence; clear cell likelihood score; contrast-enhanced ultrasound; dual-energy CT; multiparametric MRI; photon-counting detector CT; quantitative computed tomography; radiomics; renal cell carcinoma; sestamibi SPECT/CT; spectral CT.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






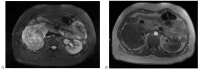

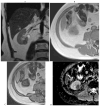

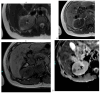

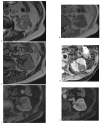

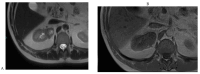

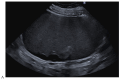

References
-
- Global Cancer Observatory: Cancer Today. WHO International Agency for Research on Cancer; Lyon, France: 2022. [(accessed on 2 May 2024)]. Available online: https://gco.iarc.fr/today.
-
- Huang J., Leung D.K., Chan E.O., Lok V., Leung S., Wong I., Lao X.Q., Zheng Z.J., Chiu P.K., Ng C.F., et al. A global trend analysis of kidney cancer incidence and mortality and their associations with smoking, alcohol consumption, and metabolic syndrome. Eur. Urol. Focus. 2022;8:200–209. doi: 10.1016/j.euf.2020.12.020. - DOI - PubMed
-
- Laguna M.P., Algaba F., Cadeddu J., Clayman R., Gill I., Gueglio G., Hohenfellner M., Joyce A., Landman J., Lee B., et al. Current patterns of presentation and treatment of renal masses: A clinical research office of the endourological society prospective study. J. Endourol. 2014;28:861–870. doi: 10.1089/end.2013.0724. - DOI - PubMed
-
- Kowalewski K.F., Egen L., Fischetti C.E., Puliatti S., Juan G.R., Taratkin M., Ines R.B., Sidoti Abate M.A., Mühlbauer J., Wessels F., et al. Artificial intelligence for renal cancer: From imaging to histology and beyond. Asian J. Urol. 2022;9:243–252. doi: 10.1016/j.ajur.2022.05.003. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

