Antisense Oligonucleotides for Rapid Translation of Gene Therapy in Glioblastoma
- PMID: 38792022
- PMCID: PMC11119631
- DOI: 10.3390/cancers16101944
Antisense Oligonucleotides for Rapid Translation of Gene Therapy in Glioblastoma
Abstract
Purpose: The limited efficacy of current treatments for malignant brain tumors necessitates novel therapeutic strategies. This study aimed to assess the potential of antisense oligonucleotides (ASOs) as adjuvant therapy for high-grade gliomas, focusing on their CNS penetration and clinical translation prospects.
Methods: A comprehensive review of the existing literature was conducted to evaluate the implications of ASOs in neuro-oncology. Studies that investigated ASO therapy's efficacy, CNS penetration, and safety profile were analyzed to assess its potential as a therapeutic intervention for high-grade gliomas.
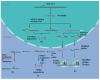
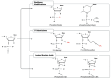
Results: ASOs present a promising avenue for enhancing targeted gene therapies in malignant gliomas. Their potent CNS penetration, in vivo durability, and efficient transduction offer advantages over conventional treatments. Preliminary in vivo and in vitro studies suggest ASOs as a viable adjuvant therapy for high-grade gliomas, warranting further exploration in clinical trials.
Conclusions: ASOs hold significant promise as adjuvant therapy for high-grade gliomas, offering improved CNS penetration and durability compared with existing treatments. While preliminary studies are encouraging, additional research is needed to establish the safety and efficacy of ASO therapy in clinical settings. Further investigation and clinical trials are warranted to validate ASOs as a transformative approach in neuro-oncology.
Keywords: adjuvant therapy; antisense oligonucleotides; blood–brain barrier; brain tumor; central nervous system; gene therapy; high-grade gliomas.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures
Similar articles
-
Therapeutic Antisense Oligonucleotides in Oncology: From Bench to Bedside.Cancers (Basel). 2024 Aug 23;16(17):2940. doi: 10.3390/cancers16172940. Cancers (Basel). 2024. PMID: 39272802 Free PMC article. Review.
-
Favorable efficacy and reduced acute neurotoxicity by antisense oligonucleotides with 2',4'-BNA/LNA with 9-(aminoethoxy)phenoxazine.Mol Ther Nucleic Acids. 2024 Mar 18;35(2):102161. doi: 10.1016/j.omtn.2024.102161. eCollection 2024 Jun 11. Mol Ther Nucleic Acids. 2024. PMID: 38978695 Free PMC article.
-
Delivery of mutant huntingtin-lowering antisense oligonucleotides to the brain by intranasally administered apolipoprotein A-I nanodisks.J Control Release. 2023 Aug;360:913-927. doi: 10.1016/j.jconrel.2023.07.027. Epub 2023 Jul 27. J Control Release. 2023. PMID: 37468110
-
Antisense oligonucleotides on neurobehavior, respiratory, and cardiovascular function, and hERG channel current studies.J Pharmacol Toxicol Methods. 2014 Jan-Feb;69(1):49-60. doi: 10.1016/j.vascn.2013.10.005. Epub 2013 Nov 8. J Pharmacol Toxicol Methods. 2014. PMID: 24211663
-
Clinical Applications of Antisense Oligonucleotides in Cancer: A Focus on Glioblastoma.Cells. 2024 Nov 11;13(22):1869. doi: 10.3390/cells13221869. Cells. 2024. PMID: 39594617 Free PMC article. Review.
Cited by
-
Gene network changes after exposure to nanoliposomes containing antisense miR-21 and miR-373 in bladder cancer Cells: An in vitro model study.Biochem Biophys Rep. 2025 May 12;42:102041. doi: 10.1016/j.bbrep.2025.102041. eCollection 2025 Jun. Biochem Biophys Rep. 2025. PMID: 40469776 Free PMC article.
-
Aptamer-mediated delivery of therapeutic oligonucleotides in glioblastoma.Transl Oncol. 2025 Oct;60:102485. doi: 10.1016/j.tranon.2025.102485. Epub 2025 Jul 26. Transl Oncol. 2025. PMID: 40716251 Free PMC article. Review.
References
-
- Weller M., van den Bent M., Tonn J.C., Stupp R., Preusser M., Cohen-Jonathan-Moyal E., Henriksson R., Le Rhun E., Balana C., Chinot O., et al. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017;18:e315–e329. doi: 10.1016/S1470-2045(17)30194-8. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

