Immunohistochemical Investigation into Protein Expression Patterns of FOXO4, IRF8 and LEF1 in Canine Osteosarcoma
- PMID: 38792023
- PMCID: PMC11120020
- DOI: 10.3390/cancers16101945
Immunohistochemical Investigation into Protein Expression Patterns of FOXO4, IRF8 and LEF1 in Canine Osteosarcoma
Abstract
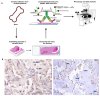
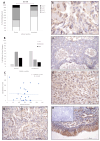
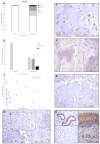
Osteosarcoma (OSA) is the most common type of primary bone malignancy in people and dogs. Our previous molecular comparisons of canine OSA against healthy bone resulted in the identification of differentially expressed protein-expressing genes (forkhead box protein O4 (FOXO4), interferon regulatory factor 8 (IRF8), and lymphoid enhancer binding factor 1 (LEF1)). Immunohistochemistry (IHC) and H-scoring provided semi-quantitative assessment of nuclear and cytoplasmic staining alongside qualitative data to contextualise staining (n = 26 patients). FOXO4 was expressed predominantly in the cytoplasm with significantly lower nuclear H-scores. IRF8 H-scores ranged from 0 to 3 throughout the cohort in the nucleus and cytoplasm. LEF1 was expressed in all patients with significantly lower cytoplasmic staining compared to nuclear. No sex or anatomical location differences were observed. While reduced levels of FOXO4 might indicate malignancy, the weak or absent protein expression limits its primary use as diagnostic tumour marker. IRF8 and LEF1 have more potential for prognostic and diagnostic uses and facilitate further understanding of their roles within their respective molecular pathways, including Wnt/beta-catenin/LEF1 signalling and differential regulation of tumour suppressor genes. Deeper understanding of the mechanisms involved in OSA are essential contributions towards the development of novel diagnostic, prognostic, and treatment options in human and veterinary medicine contexts.
Keywords: cancer identification; forkhead box protein O4; interferon regulatory factor 8; lymphoid enhancer binding factor 1; osteosarcoma; pathology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Schmidt A.F., Groenwold R.H.H., Amsellem P., Bacon N., Klungel O.H., Hoes A.W., de Boer A., Kow K., Maritato K., Kirpensteijn J., et al. Which dogs with appendicular osteosarcoma benefit most from chemotherapy after surgery? Results from an individual patient data meta-analysis. Prev. Vet. Med. 2016;125:116–125. doi: 10.1016/j.prevetmed.2015.10.016. - DOI - PubMed
-
- Greene S. Withrow & MacEwen’s Small Animal Clinical Oncology, 6th edition. Am. J. Vet. Res. 2020;81:391.
Grants and funding
LinkOut - more resources
Full Text Sources

