Heterogeneous Organocatalysts for Light-Driven Reactions in Continuous Flow
- PMID: 38792028
- PMCID: PMC11124298
- DOI: 10.3390/molecules29102166
Heterogeneous Organocatalysts for Light-Driven Reactions in Continuous Flow
Abstract
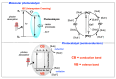
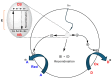
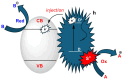
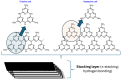
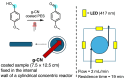
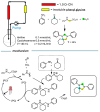
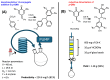
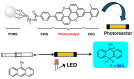
Within the realm of organic synthesis, photocatalysis has blossomed since the beginning of the last decade. A plethora of classical reactivities, such as selective oxidation of alcohol and amines, redox radical formation of reactive species in situ, and indirect activation of an organic substrate for cycloaddition by EnT, have been revised in a milder and more sustainable fashion via photocatalysis. However, even though the spark of creativity leads scientists to explore new reactions and reactivities, the urgency of replacing the toxic and critical metals that are involved as catalysts has encouraged chemists to find alternatives in the branch of science called organocatalysis. Unfortunately, replacing metal catalysts with organic analogues can be too expensive sometimes; however, this drawback can be solved by the reutilization of the catalyst if it is heterogeneous. The aim of this review is to present the recent works in the field of heterogeneous photocatalysis, applied to organic synthesis, enabled by continuous flow. In detail, among the heterogeneous catalysts, g-CN, polymeric photoactive materials, and supported molecular catalysts have been discussed within their specific sections, rather than focusing on the types of reactions.
Keywords: flow chemistry; green chemistry; heterogeneous catalysis; photocatalysis; synthetic methodologies.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



















References
-
- DeVierno Kreuder A., House-Knight T., Whitford J., Ponnusamy E., Miller P., Jesse N., Rodenborn R., Sayag S., Gebel M., Aped I., et al. A Method for Assessing Greener Alternatives between Chemical Products Following the 12 Principles of Green Chemistry. ACS Sustain. Chem. Eng. 2017;5:2927–2935. doi: 10.1021/acssuschemeng.6b02399. - DOI
-
- Di Carmine G., Abbott A.P., D’Agostino C. Deep Eutectic Solvents: Alternative Reaction Media for Organic Oxidation Reactions. React. Chem. Eng. 2021;6:582–598. doi: 10.1039/D0RE00458H. - DOI
-
- Davidson M.G., Elgie S., Parsons S., Young T.J. Production of HMF, FDCA and Their Derived Products: A Review of Life Cycle Assessment (LCA) and Techno-Economic Analysis (TEA) Studies. Green Chem. 2021;23:3154–3171. doi: 10.1039/D1GC00721A. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

