Magnetic Aerogels for Room-Temperature Catalytic Production of Bis(indolyl)methane Derivatives
- PMID: 38792085
- PMCID: PMC11124404
- DOI: 10.3390/molecules29102223
Magnetic Aerogels for Room-Temperature Catalytic Production of Bis(indolyl)methane Derivatives
Abstract
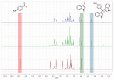
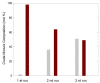
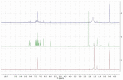
The potential of aerogels as catalysts for the synthesis of a relevant class of bis-heterocyclic compounds such as bis(indolyl)methanes was investigated. In particular, the studied catalyst was a nanocomposite aerogel based on nanocrystalline nickel ferrite (NiFe2O4) dispersed on amorphous porous silica aerogel obtained by two-step sol-gel synthesis followed by gel drying under supercritical conditions and calcination treatments. It was found that the NiFe2O4/SiO2 aerogel is an active catalyst for the selected reaction, enabling high conversions at room temperature, and it proved to be active for three repeated runs. The catalytic activity can be ascribed to both the textural and acidic features of the silica matrix and of the nanocrystalline ferrite. In addition, ferrite nanocrystals provide functionality for magnetic recovery of the catalyst from the crude mixture, enabling time-effective separation from the reaction environment. Evidence of the retention of species involved in the reaction into the catalyst is also pointed out, likely due to the porosity of the aerogel together with the affinity of some species towards the silica matrix. Our work contributes to the study of aerogels as catalysts for organic reactions by demonstrating their potential as well as limitations for the room-temperature synthesis of bis(indolyl)methanes.
Keywords: aerogel; bis(indolyl)methanes; catalyst; ferrite; nanocomposite; organic synthesis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Corrias A., Loche D., Casula M.F. Aerogels Containing Metal, Alloy, and Oxide Nanoparticles Embedded into Dielectric Matrices. In: Aegerter M.A., Leventis N., Koebel M., Steiner S.A. III, editors. Springer Handbook of Aerogels. Springer Nature; Cham, Switzerland: 2023. Springer Handbooks. - DOI
-
- Maleki H., Durães L., Portugal A. An Overview on Silica Aerogels Synthesis and Different Mechanical Reinforcing Strategies. J. Non Cryst. Solids. 2014;385:55–74. doi: 10.1016/j.jnoncrysol.2013.10.017. - DOI
-
- Pajonk G.M. Aerogel Catalysts. Appl. Catal. 1991;72:217–266. doi: 10.1016/0166-9834(91)85054-Y. - DOI
-
- Tleimat-Manzalji R., Manzalji T., Pajonk G.M. Aerogels and Xerogels for Catalytic Applications. J. Non Cryst. Solids. 1992;147–148:744–747. doi: 10.1016/S0022-3093(05)80709-4. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

