Nitrogen-Centered Radicals Derived from Azidonucleosides
- PMID: 38792171
- PMCID: PMC11124349
- DOI: 10.3390/molecules29102310
Nitrogen-Centered Radicals Derived from Azidonucleosides
Abstract
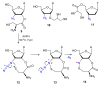
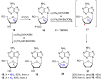
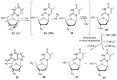
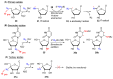
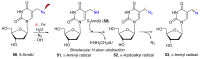
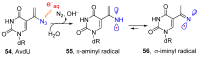
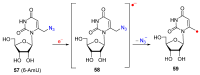
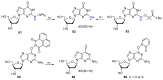
Azido-modified nucleosides have been extensively explored as substrates for click chemistry and the metabolic labeling of DNA and RNA. These compounds are also of interest as precursors for further synthetic elaboration and as therapeutic agents. This review discusses the chemistry of azidonucleosides related to the generation of nitrogen-centered radicals (NCRs) from the azido groups that are selectively inserted into the nucleoside frame along with the subsequent chemistry and biological implications of NCRs. For instance, the critical role of the sulfinylimine radical generated during inhibition of ribonucleotide reductases by 2'-azido-2'-deoxy pyrimidine nucleotides as well as the NCRs generated from azidonucleosides by radiation-produced (prehydrated and aqueous) electrons are discussed. Regio and stereoselectivity of incorporation of an azido group ("radical arm") into the frame of nucleoside and selective generation of NCRs under reductive conditions, which often produce the same radical species that are observed upon ionization events due to radiation and/or other oxidative conditions that are emphasized. NCRs generated from nucleoside-modified precursors other than azidonucleosides are also discussed but only with the direct relation to the same/similar NCRs derived from azidonucleosides.
Keywords: aminyl radicals; azides; iminyl radicals; nitrogen-centered radicals; nucleosides; purines; pyrimidines; radiation; radiosensitizers; ribonucleotide reductases.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

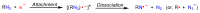

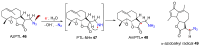
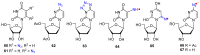



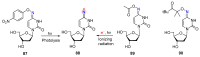

References
-
- Jiang H., Studer A. Chemistry With N-Centered Radicals Generated by Single-Electron Transfer-Oxidation Using Photoredox Catalysis. CCS Chem. 2019;1:38–49. doi: 10.31635/ccschem.019.20180026. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

