COF-SiO2@Fe3O4 Composite for Magnetic Solid-Phase Extraction of Pyrethroid Pesticides in Vegetables
- PMID: 38792172
- PMCID: PMC11123868
- DOI: 10.3390/molecules29102311
COF-SiO2@Fe3O4 Composite for Magnetic Solid-Phase Extraction of Pyrethroid Pesticides in Vegetables
Abstract
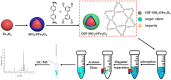
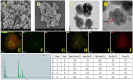
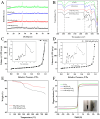
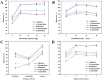
Pyrethroid pesticides (PYRs) have found widespread application in agriculture for the protection of fruit and vegetable crops. Nonetheless, excessive usage or improper application may allow the residues to exceed the safe limits and pose a threat to consumer safety. Thus, there is an urgent need to develop efficient technologies for the elimination or trace detection of PYRs from vegetables. Here, a simple and efficient magnetic solid-phase extraction (MSPE) strategy was developed for the simultaneous purification and enrichment of five PYRs in vegetables, employing the magnetic covalent organic framework nanomaterial COF-SiO2@Fe3O4 as an adsorbent. COF-SiO2@Fe3O4 was prepared by a straightforward solvothermal method, using Fe3O4 as a magnetic core and benzidine and 3,3,5,5-tetraaldehyde biphenyl as the two building units. COF-SiO2@Fe3O4 could effectively capture the targeted PYRs by virtue of its abundant π-electron system and hydroxyl groups. The impact of various experimental parameters on the extraction efficiency was investigated to optimize the MSPE conditions, including the adsorbent amount, extraction time, elution solvent type and elution time. Subsequently, method validation was conducted under the optimal conditions in conjunction with gas chromatography-mass spectrometry (GC-MS). Within the range of 5.00-100 μg·kg-1 (1.00-100 μg·kg-1 for bifenthrin and 2.5-100 μg·kg-1 for fenpropathrin), the five PYRs exhibited a strong linear relationship, with determination coefficients ranging from 0.9990 to 0.9997. The limits of detection (LODs) were 0.3-1.5 μg·kg-1, and the limits of quantification (LOQs) were 0.9-4.5 μg·kg-1. The recoveries were 80.2-116.7% with relative standard deviations (RSDs) below 7.0%. Finally, COF-SiO2@Fe3O4, NH2-SiO2@Fe3O4 and Fe3O4 were compared as MSPE adsorbents for PYRs. The results indicated that COF-SiO2@Fe3O4 was an efficient and rapid selective adsorbent for PYRs. This method holds promise for the determination of PYRs in real samples.
Keywords: covalent organic framework; gas chromatography–mass spectrometry; magnetic solid-phase extraction; pyrethroid pesticides.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
[Determination of three diphenyl ether herbicides in rice by magnetic solid phase extraction using Fe3O4@MOF-808 coupled with high performance liquid chromatography].Se Pu. 2021 Mar;39(3):316-323. doi: 10.3724/SP.J.1123.2020.06007. Se Pu. 2021. PMID: 34227312 Free PMC article. Chinese.
-
Fe3O4@COF(TAPT-DHTA) Nanocomposites as Magnetic Solid-Phase Extraction Adsorbents for Simultaneous Determination of 9 Mycotoxins in Fruits by UHPLC-MS/MS.Toxins (Basel). 2023 Feb 1;15(2):117. doi: 10.3390/toxins15020117. Toxins (Basel). 2023. PMID: 36828431 Free PMC article.
-
[Simultaneous determination of 76 pesticide residues in the traditional Chinese medicine by magnetic hydrophilic-lipophilic-balanced materials assisted matrix solid phase dispersion extraction-high performance liquid chromatography-tandem mass spectrometry].Se Pu. 2022 Apr;40(4):313-322. doi: 10.3724/SP.J.1123.2021.08014. Se Pu. 2022. PMID: 35362679 Free PMC article. Chinese.
-
Preparation of magnetic cationic Schiff base polymeric material for highly selective enrichment of avermectins from surface water and milk samples.J Chromatogr A. 2024 Aug 30;1731:465169. doi: 10.1016/j.chroma.2024.465169. Epub 2024 Jul 19. J Chromatogr A. 2024. PMID: 39043101 Review.
-
Analytical Extraction Methods and Sorbents' Development for Simultaneous Determination of Organophosphorus Pesticides' Residues in Food and Water Samples: A Review.Molecules. 2021 Sep 10;26(18):5495. doi: 10.3390/molecules26185495. Molecules. 2021. PMID: 34576966 Free PMC article. Review.
Cited by
-
[Recent progress in magnetic covalent organic framework materials for the enrichment and detection of typical organic pollutants].Se Pu. 2025 Feb;43(2):107-119. doi: 10.3724/SP.J.1123.2024.07020. Se Pu. 2025. PMID: 39844701 Free PMC article. Chinese.
References
-
- Lin X., Mou R., Cao Z., Cao Z., Chen M. Analysis of pyrethroid pesticides in Chinese vegetables and fruits by GC–MS/MS. Chem. Pap. 2018;72:1953–1962. doi: 10.1007/s11696-018-0447-1. - DOI
-
- Wang Q., Chen L., Li Y., Yang J., Yang R., Yang X. Magnetic nanocomposite-based TpPa-NO2 covalent organic framework for the extraction of pyrethroid insecticides in water, vegetable, and fruit samples. Food Anal. Methods. 2022;16:71–82. doi: 10.1007/s12161-022-02394-0. - DOI
-
- Farajzadeh M., Khoshmaram L., Nabil A. Determination of pyrethroid pesticides residues in vegetable oils using liquid–liquid extraction and dispersive liquid–liquid microextraction followed by gas chromatography–flame ionization detection. J. Food Compos. Anal. 2014;34:128–135. doi: 10.1016/j.jfca.2014.03.004. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

