Myricetin Acts as an Inhibitor of Type II NADH Dehydrogenase from Staphylococcus aureus
- PMID: 38792214
- PMCID: PMC11124336
- DOI: 10.3390/molecules29102354
Myricetin Acts as an Inhibitor of Type II NADH Dehydrogenase from Staphylococcus aureus
Abstract
Background: Staphylococcus aureus is a common pathogenic microorganism in humans and animals. Type II NADH oxidoreductase (NDH-2) is the only NADH:quinone oxidoreductase present in this organism and represents a promising target for the development of anti-staphylococcal drugs. Recently, myricetin, a natural flavonoid from vegetables and fruits, was found to be a potential inhibitor of NDH-2 of S. aureus. The objective of this study was to evaluate the inhibitory properties of myricetin against NDH-2 and its impact on the growth and expression of virulence factors in S. aureus.
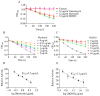
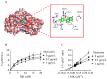
Results: A screening method was established to identify effective inhibitors of NDH-2, based on heterologously expressed S. aureus NDH-2. Myricetin was found to be an effective inhibitor of NDH-2 with a half maximal inhibitory concentration (IC50) of 2 μM. In silico predictions and enzyme inhibition kinetics further characterized myricetin as a competitive inhibitor of NDH-2 with respect to the substrate menadione (MK). The minimum inhibitory concentrations (MICs) of myricetin against S. aureus strains ranged from 64 to 128 μg/mL. Time-kill assays showed that myricetin was a bactericidal agent against S. aureus. In line with being a competitive inhibitor of the NDH-2 substrate MK, the anti-staphylococcal activity of myricetin was antagonized by MK-4. In addition, myricetin was found to inhibit the gene expression of enterotoxin SeA and reduce the hemolytic activity induced by S. aureus culture on rabbit erythrocytes in a dose-dependent manner.
Conclusions: Myricetin was newly discovered to be a competitive inhibitor of S. aureus NDH-2 in relation to the substrate MK. This discovery offers a fresh perspective on the anti-staphylococcal activity of myricetin.
Keywords: Staphylococcus aureus; Type II NADH dehydrogenase; anti-virulence activity; competitive inhibitor; myricetin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Characterization of the type 2 NADH:menaquinone oxidoreductases from Staphylococcus aureus and the bactericidal action of phenothiazines.Biochim Biophys Acta. 2014 Jul;1837(7):954-63. doi: 10.1016/j.bbabio.2014.03.017. Epub 2014 Apr 5. Biochim Biophys Acta. 2014. PMID: 24709059 Free PMC article.
-
Rhein inhibits the growth of Propionibacterium acnes by blocking NADH dehydrogenase-2 activity.J Med Microbiol. 2020 May;69(5):689-696. doi: 10.1099/jmm.0.001196. Epub 2020 May 4. J Med Microbiol. 2020. PMID: 32375980
-
Identification of new inhibitors for alternative NADH dehydrogenase (NDH-II).FEMS Microbiol Lett. 2009 Feb;291(2):157-61. doi: 10.1111/j.1574-6968.2008.01451.x. Epub 2008 Dec 5. FEMS Microbiol Lett. 2009. PMID: 19076229
-
Type-II NADH Dehydrogenase (NDH-2): a promising therapeutic target for antitubercular and antibacterial drug discovery.Expert Opin Ther Targets. 2017 Jun;21(6):559-570. doi: 10.1080/14728222.2017.1327577. Epub 2017 May 15. Expert Opin Ther Targets. 2017. PMID: 28472892 Review.
-
Targeting type II NADH dehydrogenase in tuberculosis treatment: A review.Int J Biol Macromol. 2025 May;310(Pt 4):143541. doi: 10.1016/j.ijbiomac.2025.143541. Epub 2025 Apr 26. Int J Biol Macromol. 2025. PMID: 40288271 Review.
Cited by
-
Inhibitors of NADH-O-methylquinone compound a class of antitubercular drugs.Mol Divers. 2025 Jan 25. doi: 10.1007/s11030-025-11117-6. Online ahead of print. Mol Divers. 2025. PMID: 39862351
-
Disarming Staphylococcus aureus: Review of Strategies Combating This Resilient Pathogen by Targeting Its Virulence.Pathogens. 2025 Apr 15;14(4):386. doi: 10.3390/pathogens14040386. Pathogens. 2025. PMID: 40333163 Free PMC article. Review.
-
Unlocking the Pharmacological Potential of Myricetin Against Various Pathogenesis.Int J Mol Sci. 2025 Apr 28;26(9):4188. doi: 10.3390/ijms26094188. Int J Mol Sci. 2025. PMID: 40362425 Free PMC article. Review.
-
Metabolic Derangement of Essential Transition Metals and Potential Antioxidant Therapies.Int J Mol Sci. 2024 Jul 18;25(14):7880. doi: 10.3390/ijms25147880. Int J Mol Sci. 2024. PMID: 39063122 Free PMC article. Review.
-
The protective effects and mechanism of myricetin in liver diseases (Review).Mol Med Rep. 2025 Apr;31(4):87. doi: 10.3892/mmr.2025.13452. Epub 2025 Feb 7. Mol Med Rep. 2025. PMID: 39917997 Free PMC article. Review.
References
-
- Turner N.A., Sharma-Kuinkel B.K., Maskarinec S.A., Eichenberger E.M., Shah P.P., Carugati M., Holland T.L., Fowler V.G., Jr. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019;17:203–218. doi: 10.1038/s41579-018-0147-4. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

