The Effect of Different Substances Embedded in Fullerene Cavity on Surfactant Self-Assembly Behavior through Molecular Dynamics Simulation
- PMID: 38792216
- PMCID: PMC11123870
- DOI: 10.3390/molecules29102355
The Effect of Different Substances Embedded in Fullerene Cavity on Surfactant Self-Assembly Behavior through Molecular Dynamics Simulation
Abstract
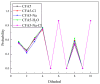
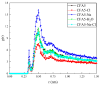
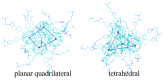
Fullerene-based amphiphiles are new types of monomers that form self-assemblies with profound applications. The conical fullerene amphiphiles (CFAs) have attracted attention for their uniquely self-assembled structures and have opened up a new field for amphiphile research. The CFAs and CFAs with different substances embedded in cavities are designed and their self-assembly behaviors are investigated using molecular dynamics (MD) simulations. The surface and internal structures of the micelles are analyzed from various perspectives, including micelle size, shape, and solvent-accessible surface area (SASA). The systems studied are all oblate micelles. In comparison, embedding Cl- or embedding Na+ in the cavities results in larger micelles and a larger deviation from the spherical shape. Two typical configurations of fullerene surfactant micelles, quadrilateral plane and tetrahedral structure, are presented. The dipole moments of the fullerene molecules are also calculated, and the results show that the embedded negatively charged Cl- leads to a decrease in the polarity of the pure fullerene molecules, while the embedded positively charged Na+ leads to an increase.
Keywords: fullerene; micelle; molecular dynamics simulations; self-assembled.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








Similar articles
-
Interfacial Chemistry of Conical Fullerene Amphiphiles in Water.Acc Chem Res. 2019 Aug 20;52(8):2090-2100. doi: 10.1021/acs.accounts.9b00318. Epub 2019 Aug 7. Acc Chem Res. 2019. PMID: 31390187
-
Giant molecular shape amphiphiles based on polystyrene-hydrophilic [60]fullerene conjugates: click synthesis, solution self-assembly, and phase behavior.J Am Chem Soc. 2012 May 9;134(18):7780-7. doi: 10.1021/ja3000529. Epub 2012 Apr 26. J Am Chem Soc. 2012. PMID: 22537051
-
Molecular dynamics simulation and thermodynamic modeling of the self-assembly of the triterpenoids asiatic acid and madecassic acid in aqueous solution.J Phys Chem B. 2008 Feb 28;112(8):2357-71. doi: 10.1021/jp074310g. Epub 2008 Feb 5. J Phys Chem B. 2008. PMID: 18247591
-
Complementary use of simulations and molecular-thermodynamic theory to model micellization.Langmuir. 2006 Feb 14;22(4):1500-13. doi: 10.1021/la052042c. Langmuir. 2006. PMID: 16460068 Review.
-
Assembly behaviors of calixarene-based amphiphile and supra-amphiphile and the applications in drug delivery and protein recognition.Adv Colloid Interface Sci. 2019 Jul;269:187-202. doi: 10.1016/j.cis.2019.04.004. Epub 2019 May 3. Adv Colloid Interface Sci. 2019. PMID: 31082545 Review.
References
-
- Kroto H.W., Heath J.R., O’Brien S.C., Curl R.F., Smalley R.E. C60: Buckminsterfullerene. Nature. 1985;318:162–163. doi: 10.1038/318162a0. - DOI
-
- Krätschmer W., Lamb L.D., Fostiropoulos K., Huffman D.R. Solid C60: A new form of carbon. Nature. 1990;347:354–358. doi: 10.1038/347354a0. - DOI
-
- Hashikawa Y., Murata Y. Cobalt-functionalized open-[60] fullerenes. Organometallics. 2024;43:227–232. doi: 10.1021/acs.organomet.3c00484. - DOI
-
- Liu F., Popov A.A. Capturing unstable metallofullerenes. Inorganics. 2024;12:48–63. doi: 10.3390/inorganics12020048. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

