Long-Term Treatment of Gaucher Disease with Velaglucerase Alfa in ERT-Naïve Patients from the Gaucher Outcome Survey (GOS) Registry
- PMID: 38792324
- PMCID: PMC11122485
- DOI: 10.3390/jcm13102782
Long-Term Treatment of Gaucher Disease with Velaglucerase Alfa in ERT-Naïve Patients from the Gaucher Outcome Survey (GOS) Registry
Abstract
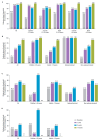
Background: Gaucher disease (GD) is a rare, autosomal, recessive condition characterized by hepatosplenomegaly, thrombocytopenia, anemia, and bone abnormalities, often requiring life-long treatment. Velaglucerase alfa has improved hematologic and visceral parameters in clinical trials; however, limited long-term efficacy and safety data are available. Methods: The Gaucher Outcome Survey (GOS), a structured and validated international registry for patients with confirmed GD, provides an opportunity to evaluate long-term data from patients receiving velaglucerase alfa. Results: This analysis included 376 treatment-naïve children and adults with GD enrolled in GOS, including 20 with type 3 GD, who initiated velaglucerase alfa through participation in clinical trials or as part of their clinical management and continued treatment for a mean (range) time of 6.6 (0.003-18.6) years. Initial improvements in hematologic and visceral parameters and the biomarkers glucosylsphingosine (lyso-GL1) and chitotriosidase were observed after one year of treatment and were maintained throughout the follow-up period. Of 129 (34.3%) patients who developed adverse events during the follow-up period, events were considered related to treatment in 33 (8.8%). None led to treatment discontinuation. There were 21 deaths overall, none of which were considered related to treatment. Conclusions: This analysis of data from the GOS registry supports the safety and efficacy of velaglucerase alfa in patients with GD.
Keywords: ERT; GOS; Gaucher disease; enzyme replacement therapy; long term; registry; velaglucerase alfa.
Conflict of interest statement
Patrick Deegan received Speaker Honoraria from Takeda, institutional research support from Takeda, consulting fees from Sanofi, and institutional research support from Sanofi Genzyme. Heather Lau has received consulting fees from Actelion, Pfizer, Sanofi Genzyme, and Shire/Takeda, and research grants from Amicus, BioMarin, Pfizer, Protalix, Sangamo, Sanofi Genzyme, Shire/Takeda, and Ultragenyx. Deborah Elstein was a paid consultant for Takeda at the time the analysis was conducted. Diego Fernandez-Sasso and Majdolen Istaiti have no financial disclosures to declare. Pilar Giraldo receives financial compensation for presentations, advisory boards, and research projects from Pfizer, Sanofi Genzyme, and Takeda. The financial contributions are destined to research in Gaucher disease and other lysosomal diseases through the FEETEG. Derralynn Hughes has received consulting fees and fees for non-CME/CE services from Genzyme, Sanofi, and Takeda. Ari Zimran receives honoraria from Takeda, Pfizer, and BioEvents, and consultancy fees from Takeda, Prevail therapeutics, NLC Pharma, and Insightec. Jaco Botha and Noga Gadir are employees of Takeda and own stocks or stock options in Takeda. Shoshana Revel-Vilk receives grant/research support, honoraria, and advisory fees from Takeda, Pfizer, and Sanofi/Genzyme. The Shaare Zedek Medical Center Gaucher Unit receives support from Sanofi Genzyme for participation in the ICGG Registry, from Takeda for the GOS Registry, and Pfizer for Taliglucerase Alfa Surveillance. The Unit also receives research grants from Takeda, Pfizer, Sanofi Genzyme, and Centogene.
Figures
References
-
- Stirnemann J., Belmatoug N., Camou F., Serratrice C., Froissart R., Caillaud C., Levade T., Astudillo L., Serratrice J., Brassier A., et al. A review of Gaucher disease pathophysiology, clinical presentation and treatments. Int. J. Mol. Sci. 2017;18:441. doi: 10.3390/ijms18020441. - DOI - PMC - PubMed
-
- Revel-Vilk S., Szer J., Zimran A. Gaucher disease and related lysosomal storage diseases. In: Kaushansky K., Prchal J.T., Burns L.J., Lichtman M.A., Levi M., Linch D.C., editors. Williams Hematology. 10th ed. McGraw-Hill; New York, NY, USA: 2021.
-
- Elstein D., Mehta A., Hughes D.A., Giraldo P., Charrow J., Smith L., Shankar S.P., Hangartner T.N., Kunes Y., Wang N., et al. Safety and efficacy results of switch from imiglucerase to velaglucerase alfa treatment in patients with type 1 Gaucher disease. Am. J. Hematol. 2015;90:592–597. doi: 10.1002/ajh.24007. - DOI - PubMed
-
- Elstein D., Mellgard B., Dinh Q., Lan L., Qiu Y., Cozma C., Eichler S., Bottcher T., Zimran A. Reductions in glucosylsphingosine (lyso-Gb1) in treatment-naive and previously treated patients receiving velaglucerase alfa for type 1 Gaucher disease: Data from phase 3 clinical trials. Mol. Genet. Metab. 2017;122:113–120. doi: 10.1016/j.ymgme.2017.08.005. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

