Blood Purification in Hepatic Dysfunction after Liver Transplant or Extensive Hepatectomy: Far from the Best-Case Scenarios
- PMID: 38792395
- PMCID: PMC11122492
- DOI: 10.3390/jcm13102853
Blood Purification in Hepatic Dysfunction after Liver Transplant or Extensive Hepatectomy: Far from the Best-Case Scenarios
Abstract
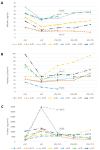
Background: Hepatic dysfunction (HD) after liver transplantation (LT) or extended hepatic resection (EHR) is associated with graft failure and high short-term mortality. We evaluated the safety and depurative efficacy of CytoSorb® in these settings. The primary endpoint was the change in serum total bilirubin at the end of the treatment compared to the baseline value. The secondary endpoint was to evaluate the trend of serum total bilirubin and coagulation parameters up to 72 h after discontinuation of CytoSorb®. The effects of CytoSorb® therapy on the degree of hepatic encephalopathy (HE), Sequential Organ Failure Assessment (SOFA), and Model for End-Stage Liver Disease (MELD) scores as well as the hemodynamic status compared to baseline were also assessed. Methods: Adult patients with a serum total bilirubin level > 10 mg/dL admitted to the Intensive Care Unit were included. Exclusion criteria were hemodynamic instability, postoperative bleeding and platelet count < 20,000/mm3. Results: Seven patients were treated. Serum total bilirubin was significantly reduced at the end of treatment. However, seventy-two hours after the discontinuation of extracorporeal therapy, bilirubin levels returned to baseline levels in four patients. A decrease in platelet count was found during therapy, and platelet transfusion was required in six cases. A significant increase in D-dimer at the end of treatment was detected. HE degree, SOFA and MELD scores remained stable, while a deterioration in hemodynamic status was observed in two cases. Conclusions: Our preliminary findings did not show the possible benefits of CytoSorb® in rebalancing clinical and laboratory parameters in patients with HD after LT or EHR.
Keywords: graft failure; hemoadsorption; liver dysfunction; liver resection; liver transplant.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Multicentered prospective investigator initiated study to evaluate the clinical outcomes with extracorporeal cytokine adsorption device (CytoSorb®) in patients with sepsis and septic shock.World J Crit Care Med. 2021 Jan 9;10(1):22-34. doi: 10.5492/wjccm.v10.i1.22. eCollection 2021 Jan 9. World J Crit Care Med. 2021. PMID: 33505870 Free PMC article.
-
Hemoadsorption in 'Liver Indication'-Analysis of 109 Patients' Data from the CytoSorb International Registry.J Clin Med. 2021 Nov 5;10(21):5182. doi: 10.3390/jcm10215182. J Clin Med. 2021. PMID: 34768702 Free PMC article.
-
Artificial Liver Support with CytoSorb and MARS in Liver Failure: A Retrospective Propensity Matched Analysis.J Clin Med. 2023 Mar 14;12(6):2258. doi: 10.3390/jcm12062258. J Clin Med. 2023. PMID: 36983259 Free PMC article.
-
Predictive factors of short term outcome after liver transplantation: A review.World J Gastroenterol. 2016 Jul 14;22(26):5936-49. doi: 10.3748/wjg.v22.i26.5936. World J Gastroenterol. 2016. PMID: 27468188 Free PMC article. Review.
-
Should patients with acute-on-chronic liver failure grade 3 receive higher priority for liver transplantation?J Hepatol. 2023 Jun;78(6):1118-1123. doi: 10.1016/j.jhep.2022.12.026. J Hepatol. 2023. PMID: 37208098 Review.
Cited by
-
Reply to Riva et al. Comment on "Gaspari et al. Blood Purification in Hepatic Dysfunction after Liver Transplant or Extensive Hepatectomy: Far from the Best-Case Scenarios. J. Clin. Med. 2024, 13, 2853".J Clin Med. 2025 Jan 27;14(3):822. doi: 10.3390/jcm14030822. J Clin Med. 2025. PMID: 39941493 Free PMC article.
-
Comment on Gaspari et al. Blood Purification in Hepatic Dysfunction after Liver Transplant or Extensive Hepatectomy: Far from the Best-Case Scenarios. J. Clin. Med. 2024, 13, 2853.J Clin Med. 2025 Jan 23;14(3):716. doi: 10.3390/jcm14030716. J Clin Med. 2025. PMID: 39941387 Free PMC article.
-
Extracorporeal therapies for post-liver transplant recipient: The road less traveled.World J Transplant. 2025 Sep 18;15(3):101975. doi: 10.5500/wjt.v15.i3.101975. World J Transplant. 2025. PMID: 40881747 Review.
References
-
- Moosburner S., Wiering L., Roschke N.N., Winter A., Demir M., Gaßner J.M.G.V., Zimmer M., Ritschl P., Globke B., Lurje G., et al. Validation of risk scores for allograft failure after liver transplantation in Germany: A retrospective cohort analysis. Hepatol. Commun. 2023;7:e0012. doi: 10.1097/HC9.0000000000000012. - DOI - PMC - PubMed
-
- Rahbari N.N., Garden O.J., Padbury R., Brooke-Smith M., Crawford M., Adam R., Koch M., Makuuchi M., Dematteo R.P., Christophi C., et al. Posthepatectomy liver failure: A definition and grading by the International Study Group of Liver Surgery (ISGLS) Surgery. 2011;149:713–724. doi: 10.1016/j.surg.2010.10.001. - DOI - PubMed
-
- Gaspari R., Cavaliere F., Sollazzi L., Perilli V., Melchionda I., Agnes S., Gasbarrini A., Avolio A.W. Molecular adsorbent recirculating system (Mars) in patients with primary nonfunction and other causes of graft dysfunction after liver transplantation in the era of extended criteria donor organs. Transplant. Proc. 2009;41:253–258. doi: 10.1016/j.transproceed.2008.10.066. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

