Intraoperative Neurophysiological Monitoring in Neurosurgery
- PMID: 38792507
- PMCID: PMC11122101
- DOI: 10.3390/jcm13102966
Intraoperative Neurophysiological Monitoring in Neurosurgery
Abstract
Intraoperative neurophysiological monitoring (IONM) is a crucial advancement in neurosurgery, enhancing procedural safety and precision. This technique involves continuous real-time assessment of neurophysiological signals, aiding surgeons in timely interventions to protect neural structures. In addition to inherent limitations, IONM necessitates a detailed anesthetic plan for accurate signal recording. Given the growing importance of IONM in neurosurgery, we conducted a narrative review including the most relevant studies about the modalities and their application in different fields of neurosurgery. In particular, this review provides insights for all physicians and healthcare professionals unfamiliar with IONM, elucidating commonly used techniques in neurosurgery. In particular, it discusses the roles of IONM in various neurosurgical settings such as tumoral brain resection, neurovascular surgery, epilepsy surgery, spinal surgery, and peripheral nerve surgery. Furthermore, it offers an overview of the anesthesiologic strategies and limitations of techniques essential for the effective implementation of IONM.
Keywords: direct cortical stimulation; electrocorticography; electromyography; evoked potentials; intraoperative neurophysiological monitoring; neurosurgery.
Conflict of interest statement
Federico Longhini contributed to the development of a new helmet for mechanical ventilation and he is designated as the inventor (European Patent number 3320941) not related to the present manuscript. He also received speaking fees from Draeger, Intersurgical, and Fisher & Paykel. The remaining authors have no relevant financial or non-financial interests to disclose.
Figures
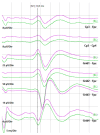
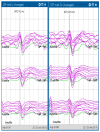



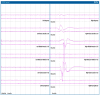
References
-
- Moehl K., Shandal V., Anetakis K., Paras S., Mina A., Crammond D., Balzer J., Thirumala P.D. Predicting transient ischemic attack after carotid endarterectomy: The role of intraoperative neurophysiological monitoring. Clin. Neurophysiol. 2022;141:1–8. doi: 10.1016/j.clinph.2022.06.010. - DOI - PubMed
-
- Szelenyi A., Fernandez-Conejero I., Kodama K. Surgery and intraoperative neurophysiologic monitoring for aneurysm clipping. Handb. Clin. Neurol. 2022;186:375–393. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

