Evolocumab Treatment in Dyslipidemic Patients Undergoing Coronary Artery Bypass Grafting: One-Year Safety and Efficacy Results
- PMID: 38792527
- PMCID: PMC11121999
- DOI: 10.3390/jcm13102987
Evolocumab Treatment in Dyslipidemic Patients Undergoing Coronary Artery Bypass Grafting: One-Year Safety and Efficacy Results
Abstract
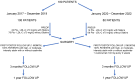
Background: The inhibition of PCSK9 lowered LDL cholesterol levels, reducing the risk of cardiovascular events. However, the effect on patients who have undergone surgical myocardial revascularization has not yet been evaluated. Methods: From January 2017 to December 2022, 180 dyslipidemic patients who underwent coronary artery bypass were included in the study. Until December 2019, 100 patients optimized therapy with statin ± ezetimibe (SG). Since January 2020, 80 matched patients added treatment with Evolocumab every 2 weeks (EG). All 180 patients were followed-up at 3 and 12 months, comparing outcomes. Results: The two groups are homogenous. At 3 months and 1 year, a significant decrease in the parameter mean levels of LDL cholesterol and total cholesterol is detected in the Evolocumab group compared to the standard group. No mortality was detected in either group. No complications or drug discontinuation were recorded. In the SG group, five patients (5%) suffered a myocardial infarction during the 1-year follow-up. In the EG group, two patients (2.5%) underwent PTCA due to myocardial infarction. There is no significant difference in overall survival according to the new treatment (p-value = 0.9), and the hazard ratio is equal to 0.94 (95% C.I.: [0.16-5.43]; p-value = 0.9397). Conclusions: The use of Evolocumab, which was started immediately after coronary artery bypass graft surgery, significantly reduced LDL cholesterol and total cholesterol levels compared to statin treatment alone and is completely safe. However, at one year of follow-up, this result did not have impact on the reduction in major clinical events.
Keywords: atherosclerosis; coronary artery bypass grafting; dyslipidemia.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Robinson J.G., Nedergaard B.S., Rogers W.J., Fialkow J., Neutel J.M., Ramstad D., Somaratne R., Legg J.C., Nelson P., Scott R., et al. Effect of Evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: The LAPLACE-2 randomized clinical trial. JAMA. 2014;311:1870–1882. doi: 10.1001/jama.2014.4030. - DOI - PubMed
-
- Koren M.J., Lundqvist P., Bolognese M., Neutel J.M., Monsalvo M.L., Yang J., Kim J.B., Scott R., Wasserman S.M., Bays H., et al. Anti-PCSK9 monotherapy for hypercholesterolemia: The MENDEL-2 randomized, controlled phase III clinical trial of Evolocumab. J. Am. Coll. Cardiol. 2014;63:2531–2540. doi: 10.1016/j.jacc.2014.03.018. - DOI - PubMed
-
- Stroes E., Colquhoun D., Sullivan D., Civeira F., Rosenson R.S., Watts G.F., Bruckert E., Cho L., Dent R., Knusel B., et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: The GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of Evolocumab. J. Am. Coll. Cardiol. 2014;63:2541–2548. doi: 10.1016/j.jacc.2014.03.019. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

